Acute and Chronic Electroconvulsive Seizures (ECS) Differentially Regulate the Expression of Epigenetic Machinery in the Adult Rat Hippocampus
- PMID: 27207907
- PMCID: PMC5043647
- DOI: 10.1093/ijnp/pyw040
Acute and Chronic Electroconvulsive Seizures (ECS) Differentially Regulate the Expression of Epigenetic Machinery in the Adult Rat Hippocampus
Abstract
Background: Electroconvulsive seizure treatment is a fast-acting antidepressant therapy that evokes rapid transcriptional, neurogenic, and behavioral changes. Epigenetic mechanisms contribute to altered gene regulation, which underlies the neurogenic and behavioral effects of electroconvulsive seizure. We hypothesized that electroconvulsive seizure may modulate the expression of epigenetic machinery, thus establishing potential alterations in the epigenetic landscape.
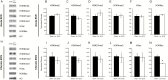
Methods: We examined the influence of acute and chronic electroconvulsive seizure on the gene expression of histone modifiers, namely histone acetyltransferases, histone deacetylases, histone methyltransferases, and histone (lysine) demethylases as well as DNA modifying enzymes, including DNA methyltransferases, DNA demethylases, and methyl-CpG-binding proteins in the hippocampi of adult male Wistar rats using quantitative real time-PCR analysis. Further, we examined the influence of acute and chronic electroconvulsive seizure on global and residue-specific histone acetylation and methylation levels within the hippocampus, a brain region implicated in the cellular and behavioral effects of electroconvulsive seizure.
Results: Acute and chronic electroconvulsive seizure induced a primarily unique, and in certain cases bidirectional, regulation of histone and DNA modifiers, and methyl-CpG-binding proteins, with an overlapping pattern of gene regulation restricted to Sirt4, Mll3, Jmjd3, Gadd45b, Tet2, and Tet3. Global histone acetylation and methylation levels were predominantly unchanged, with the exception of a significant decline in H3K9 acetylation in the hippocampus following chronic electroconvulsive seizure.
Conclusions: Electroconvulsive seizure treatment evokes the transcriptional regulation of several histone and DNA modifiers, and methyl-CpG-binding proteins within the hippocampus, with a predominantly distinct pattern of regulation induced by acute and chronic electroconvulsive seizure.
Keywords: DNA demethylase; DNA methyltransferase; histone acetyltransferase; histone deacetylase; histone demethylase; histone methyltransferase; methyl-CpG-binding proteins.
© The Author 2016. Published by Oxford University Press on behalf of CINP.
Figures




Similar articles
-
Early stress evokes dysregulation of histone modifiers in the medial prefrontal cortex across the life span.Dev Psychobiol. 2016 Mar;58(2):198-210. doi: 10.1002/dev.21365. Epub 2015 Sep 23. Dev Psychobiol. 2016. PMID: 26395029
-
Epigenetic landscape of amphetamine and methamphetamine addiction in rodents.Epigenetics. 2015;10(7):574-80. doi: 10.1080/15592294.2015.1055441. Epigenetics. 2015. PMID: 26023847 Free PMC article. Review.
-
Developmental exposure to 50 parts-per-billion arsenic influences histone modifications and associated epigenetic machinery in a region- and sex-specific manner in the adult mouse brain.Toxicol Appl Pharmacol. 2015 Oct 1;288(1):40-51. doi: 10.1016/j.taap.2015.07.013. Epub 2015 Jul 17. Toxicol Appl Pharmacol. 2015. PMID: 26193056 Free PMC article.
-
Uteroplacental insufficiency affects epigenetic determinants of chromatin structure in brains of neonatal and juvenile IUGR rats.Physiol Genomics. 2006 Mar 13;25(1):16-28. doi: 10.1152/physiolgenomics.00093.2005. Epub 2005 Dec 27. Physiol Genomics. 2006. PMID: 16380407
-
Epigenetic targeting of histone deacetylase: therapeutic potential in Parkinson's disease?Pharmacol Ther. 2013 Oct;140(1):34-52. doi: 10.1016/j.pharmthera.2013.05.010. Epub 2013 May 24. Pharmacol Ther. 2013. PMID: 23711791 Review.
Cited by
-
Electroconvulsive Seizures in Rats and Fractionation of Their Hippocampi to Examine Seizure-induced Changes in Postsynaptic Density Proteins.J Vis Exp. 2017 Aug 15;(126):56016. doi: 10.3791/56016. J Vis Exp. 2017. PMID: 28829421 Free PMC article.
-
Exome sequencing of 20,979 individuals with epilepsy reveals shared and distinct ultra-rare genetic risk across disorder subtypes.medRxiv [Preprint]. 2024 Sep 20:2023.02.22.23286310. doi: 10.1101/2023.02.22.23286310. medRxiv. 2024. Update in: Nat Neurosci. 2024 Oct;27(10):1864-1879. doi: 10.1038/s41593-024-01747-8. PMID: 36865150 Free PMC article. Updated. Preprint.
-
Volume of the Human Hippocampus and Clinical Response Following Electroconvulsive Therapy.Biol Psychiatry. 2018 Oct 15;84(8):574-581. doi: 10.1016/j.biopsych.2018.05.017. Epub 2018 May 29. Biol Psychiatry. 2018. PMID: 30006199 Free PMC article.
-
A systematic mini-review of epigenetic mechanisms associated with electroconvulsive therapy in humans.Front Hum Neurosci. 2023 Mar 9;17:1143332. doi: 10.3389/fnhum.2023.1143332. eCollection 2023. Front Hum Neurosci. 2023. PMID: 36968786 Free PMC article. Review.
-
Decoding the Mechanism of Action of Rapid-Acting Antidepressant Treatment Strategies: Does Gender Matter?Int J Mol Sci. 2019 Feb 22;20(4):949. doi: 10.3390/ijms20040949. Int J Mol Sci. 2019. PMID: 30813226 Free PMC article. Review.
References
-
- Bhaskara S, Knutson SK, Jiang G, Chandrasekharan MB, Wilson AJ, Zheng S, Yenamandra A, Locke K, Yuan JL, Bonine-Summers AR, Wells CE, Kaiser JF, Washington MK, Zhao Z, Wagner FF, Sun ZW, Xia F, Holson EB, Khabele D, Hiebert SW. (2010. ) Hdac3 is essential for the maintenance of chromatin structure and genome stability. Cancer Cell 18:436–447. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

