Angiotensin-Converting Enzyme Inhibitor Initiation and Dose Uptitration in Children With Cardiovascular Disease: A Retrospective Review of Standard Clinical Practice and a Prospective Randomized Clinical Trial
- PMID: 27207965
- PMCID: PMC4889193
- DOI: 10.1161/JAHA.116.003230
Angiotensin-Converting Enzyme Inhibitor Initiation and Dose Uptitration in Children With Cardiovascular Disease: A Retrospective Review of Standard Clinical Practice and a Prospective Randomized Clinical Trial
Erratum in
-
Angiotensin-Converting Enzyme Inhibitor Initiation and Dose Uptitration in Children With Cardiovascular Disease: A Retrospective Review of Standard Clinical Practice and a Prospective Randomized Clinical Trial.J Am Heart Assoc. 2016 Jun 16;5(6):e002067. doi: 10.1161/JAHA.116.002067. J Am Heart Assoc. 2016. PMID: 27312801 Free PMC article. No abstract available.
Abstract
Background: Angiotensin-converting enzyme inhibitors (ACEIs) are a mainstay of medical management in pediatric cardiology. However, there are no data defining how best to initiate and uptitrate the dose of these medications in children.
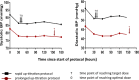
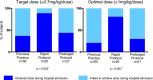
Methods and results: Retrospective chart review revealed only 24% of our pediatric cardiology inpatients were discharged on predefined optimal doses of ACEIs and few underwent further dose uptitration in the 8 weeks after hospital discharge. Therefore, 2 alternative protocols for initiation of captopril were compared in a prospective randomized clinical trial. A "rapid uptitration" protocol reached an optimal dose on day 3, whereas the alternative, "prolonged uptitration" protocol, reached an optimal dose on day 9. Forty-6 patients (54% male) were recruited to the trial, with a median age of 0.7 year (IQR 0.5-2.3 years). Captopril was initiated while in intensive care in 39% of patients and on the cardiology ward in 61%. There were no differences between the protocols in episodes of hypotension, symptomatic hypotension, or indices of renal function. Patients following the rapid protocol reached higher doses of captopril (0.93±0.24 versus 0.57±0.38 mg/kg per dose, P<0.0001) and were more likely to have achieved the predefined target (88% versus 43%, P=0.002) and optimal ACEI doses (80% versus 29%, P=0.001) before discharge.
Conclusions: A protocol of rapid ACEI dose uptitration for infants and children with cardiovascular disease can be introduced safely, even in patients receiving intensive care therapy. Compared with standard clinical practice or with a more prolonged protocol, rapid ACEI dose uptitration achieves a higher dosage in this population with no evident disadvantages.
Clinical trial registration: URL: https://www.clinicaltrials.gov/. Unique identifier: NCT00742040.
Keywords: congenital; drugs; heart defects; heart failure; pediatrics.
© 2016 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures

References
-
- Kantor PF, Lougheed J, Dancea A, McGillion M. Presentation, diagnosis, and medical management of heart failure in children: Canadian Cardiovascular Society guidelines. Can J Cardiol. 2013;29:1535–1552. - PubMed
-
- Hsu DT, Pearson GD. Heart failure in children: part II: diagnosis, treatment, and future directions. Circ Heart Fail. 2009;2:490–498. - PubMed
-
- Kirk R, Dipchand AI, Rosenthal DN, et al. The International Society for Heart and Lung Transplantation Guidelines for the management of pediatric heart failure: Executive summary [Corrected]. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation 2014;33:888–909. - PubMed
-
- Momma K. ACE inhibitors in pediatric patients with heart failure. Paediatr Drugs. 2006;8:55–69. - PubMed
-
- Duboc D, Meune C, Pierre B, Wahbi K, Eymard B, Toutain A, Berard C, Vaksmann G, Weber S, Bécane H‐M. Perindopril preventive treatment on mortality in Duchenne muscular dystrophy: 10 years’ follow‐up. Am Heart J. 2007;154:596–602. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

