The neprilysin pathway in heart failure: a review and guide on the use of sacubitril/valsartan
- PMID: 27207980
- PMCID: PMC5013095
- DOI: 10.1136/heartjnl-2014-306775
The neprilysin pathway in heart failure: a review and guide on the use of sacubitril/valsartan
Abstract
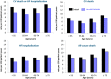
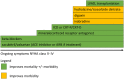
Inhibition of neurohumoural pathways such as the renin angiotensin aldosterone and sympathetic nervous systems is central to the understanding and treatment of heart failure (HF). Conversely, until recently, potentially beneficial augmentation of neurohumoural systems such as the natriuretic peptides has had limited therapeutic success. Administration of synthetic natriuretic peptides has not improved outcomes in acute HF but modulation of the natriuretic system through inhibition of the enzyme that degrades natriuretic (and other vasoactive) peptides, neprilysin, has proven to be successful. After initial failures with neprilysin inhibition alone or dual neprilysin-angiotensin converting enzyme (ACE) inhibition, the Prospective comparison of angiotensin receptor neprilysin inhibitor (ARNI) with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF) trial demonstrated that morbidity and mortality can be improved with the angiotensin receptor blocker neprilysin inhibitor sacubitril/valsartan (formerly LCZ696). In comparison to the ACE inhibitor enalapril, sacubitril/valsartan reduced the occurrence of the primary end point (cardiovascular death or hospitalisation for HF) by 20% with a 16% reduction in all-cause mortality. These findings suggest that sacubitril/valsartan should replace an ACE inhibitor or angiotensin receptor blocker as the foundation of treatment of symptomatic patients (NYHA II-IV) with HF and a reduced ejection fraction. This review will explore the background to neprilysin inhibition in HF, the results of the PARADIGM-HF trial and offer guidance on how to use sacubitril/valsartan in clinical practice.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
