Editor's Highlight: Analysis of the Effects of Cell Stress and Cytotoxicity on In Vitro Assay Activity Across a Diverse Chemical and Assay Space
- PMID: 27208079
- PMCID: PMC6280881
- DOI: 10.1093/toxsci/kfw092
Editor's Highlight: Analysis of the Effects of Cell Stress and Cytotoxicity on In Vitro Assay Activity Across a Diverse Chemical and Assay Space
Erratum in
-
Analysis of the Effects of Cell Stress and Cytotoxicity on In Vitro Assay Activity Across a Diverse Chemical and Assay Space.Toxicol Sci. 2016 Oct;153(2):409. doi: 10.1093/toxsci/kfw148. Epub 2016 Sep 7. Toxicol Sci. 2016. PMID: 27605417 Free PMC article. No abstract available.
Abstract
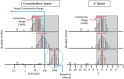
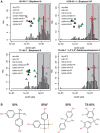
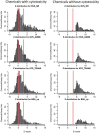
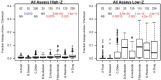
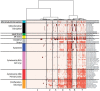
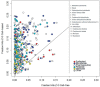
Chemical toxicity can arise from disruption of specific biomolecular functions or through more generalized cell stress and cytotoxicity-mediated processes. Here, responses of 1060 chemicals including pharmaceuticals, natural products, pesticidals, consumer, and industrial chemicals across a battery of 815 in vitro assay endpoints from 7 high-throughput assay technology platforms were analyzed in order to distinguish between these types of activities. Both cell-based and cell-free assays showed a rapid increase in the frequency of responses at concentrations where cell stress/cytotoxicity responses were observed in cell-based assays. Chemicals that were positive on at least 2 viability/cytotoxicity assays within the concentration range tested (typically up to 100 μM) activated a median of 12% of assay endpoints whereas those that were not cytotoxic in this concentration range activated 1.3% of the assays endpoints. The results suggest that activity can be broadly divided into: (1) specific biomolecular interactions against one or more targets (eg, receptors or enzymes) at concentrations below which overt cytotoxicity-associated activity is observed; and (2) activity associated with cell stress or cytotoxicity, which may result from triggering specific cell stress pathways, chemical reactivity, physico-chemical disruption of proteins or membranes, or broad low-affinity non-covalent interactions. Chemicals showing a greater number of specific biomolecular interactions are generally designed to be bioactive (pharmaceuticals or pesticidal active ingredients), whereas intentional food-use chemicals tended to show the fewest specific interactions. The analyses presented here provide context for use of these data in ongoing studies to predict in vivo toxicity from chemicals lacking extensive hazard assessment.
Keywords: In vitro; cell stress; cytotoxicity; high-throughput screening; oxidative stress.
Published by Oxford University Press on behalf of the Society of Toxicology 2016. This work is written by US Government employees and is in the public domain in the US.
Figures
References
-
- Akaike H. (1998). Information Theory and an Extension of the Maximum Likelihood Principle. Springer, New York.
-
- Ankley G. T., Bennett R. S., Erickson R. J., Hoff D. J., Hornung M. W., Johnson R. D., Mount D. R., Nichols J. W., Russom C. L., Schmieder P. K., et al. (2010). Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 29, 730–741. - PubMed
-
- Attene-Ramos M. S., Huang R., Michael S., Witt K. L., Richard A., Tice R. R., Simeonov A., Austin C. P., Xia M. (2015). Profiling of the Tox21 chemical collection for mitochondrial function to identify compounds that acutely decrease mitochondrial membrane potential. Environ. Health Perspect. 123, 49–56. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

