A Novel Glycoside Hydrolase Family 5 β-1,3-1,6-Endoglucanase from Saccharophagus degradans 2-40T and Its Transglycosylase Activity
- PMID: 27208098
- PMCID: PMC4959192
- DOI: 10.1128/AEM.00635-16
A Novel Glycoside Hydrolase Family 5 β-1,3-1,6-Endoglucanase from Saccharophagus degradans 2-40T and Its Transglycosylase Activity
Abstract
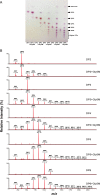
In this study, we characterized Gly5M, originating from a marine bacterium, as a novel β-1,3-1,6-endoglucanase in glycoside hydrolase family 5 (GH5) in the Carbohydrate-Active enZyme database. The gly5M gene encodes Gly5M, a newly characterized enzyme from GH5 subfamily 47 (GH5_47) in Saccharophagus degradans 2-40(T) The gly5M gene was cloned and overexpressed in Escherichia coli Through analysis of the enzymatic reaction products by thin-layer chromatography, high-performance liquid chromatography, and matrix-assisted laser desorption ionization-tandem time of flight mass spectrometry, Gly5M was identified as a novel β-1,3-endoglucanase (EC 3.2.1.39) and bacterial β-1,6-glucanase (EC 3.2.1.75) in GH5. The β-1,3-endoglucanase and β-1,6-endoglucanase activities were detected by using laminarin (a β-1,3-glucan with β-1,6-glycosidic linkages derived from brown macroalgae) and pustulan (a β-1,6-glucan derived from fungal cell walls) as the substrates, respectively. This enzyme also showed transglycosylase activity toward β-1,3-oligosaccharides when laminarioligosaccharides were used as the substrates. Since laminarin is the major form of glucan storage in brown macroalgae, Gly5M could be used to produce glucose and laminarioligosaccharides, using brown macroalgae, for industrial purposes.
Importance: In this study, we have discovered a novel β-1,3-1,6-endoglucanase with a unique transglycosylase activity, namely, Gly5M, from a marine bacterium, Saccharophagus degradans 2-40(T) Gly5M was identified as the newly found β-1,3-endoglucanase and bacterial β-1,6-glucanase in GH5. Gly5M is capable of cleaving glycosidic linkages of both β-1,3-glucans and β-1,6-glucans. Gly5M also possesses a transglycosylase activity toward β-1,3-oligosacchrides. Due to the broad specificity of Gly5M, this enzyme can be used to produce glucose or high-value β-1,3- and/or β-1,6-oligosaccharides.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures





Similar articles
-
The first bacterial β-1,6-endoglucanase from Saccharophagus degradans 2-40T for the hydrolysis of pustulan and laminarin.Appl Microbiol Biotechnol. 2017 Jan;101(1):197-204. doi: 10.1007/s00253-016-7753-8. Epub 2016 Aug 12. Appl Microbiol Biotechnol. 2017. PMID: 27521023
-
The Quaternary Structure of a Glycoside Hydrolase Dictates Specificity toward β-Glucans.J Biol Chem. 2016 Mar 25;291(13):7183-94. doi: 10.1074/jbc.M115.695999. Epub 2016 Jan 11. J Biol Chem. 2016. PMID: 26755730 Free PMC article.
-
Specific hydrolysis of curdlan with a novel glycoside hydrolase family 128 β-1,3-endoglucanase containing a carbohydrate-binding module.Carbohydr Polym. 2021 Feb 1;253:117276. doi: 10.1016/j.carbpol.2020.117276. Epub 2020 Oct 22. Carbohydr Polym. 2021. PMID: 33278947
-
Review: The structure and function of cellulase (endo-β-1,4-glucanase) and hemicellulase (β-1,3-glucanase and endo-β-1,4-mannase) enzymes in invertebrates that consume materials ranging from microbes, algae to leaf litter.Comp Biochem Physiol B Biochem Mol Biol. 2020 Feb;240:110354. doi: 10.1016/j.cbpb.2019.110354. Epub 2019 Oct 21. Comp Biochem Physiol B Biochem Mol Biol. 2020. PMID: 31647988 Review.
-
Carbohydrase systems of Saccharophagus degradans degrading marine complex polysaccharides.Mar Drugs. 2011;9(4):645-665. doi: 10.3390/md9040645. Epub 2011 Apr 21. Mar Drugs. 2011. PMID: 21731555 Free PMC article. Review.
Cited by
-
Update on Marine Carbohydrate Hydrolyzing Enzymes: Biotechnological Applications.Molecules. 2018 Apr 13;23(4):901. doi: 10.3390/molecules23040901. Molecules. 2018. PMID: 29652849 Free PMC article. Review.
-
Advances in oligosaccharides production from brown seaweeds: extraction, characterization, antimetabolic syndrome, and other potential applications.Bioengineered. 2023 Dec;14(1):2252659. doi: 10.1080/21655979.2023.2252659. Epub 2023 Sep 19. Bioengineered. 2023. PMID: 37726874 Free PMC article. Review.
-
Brown Algae Carbohydrates: Structures, Pharmaceutical Properties, and Research Challenges.Mar Drugs. 2021 Oct 31;19(11):620. doi: 10.3390/md19110620. Mar Drugs. 2021. PMID: 34822491 Free PMC article. Review.
-
Carbohydrate-Binding Module and Linker Allow Cold Adaptation and Salt Tolerance of Maltopentaose-Forming Amylase From Marine Bacterium Saccharophagus degradans 2-40 T.Front Microbiol. 2021 Jul 14;12:708480. doi: 10.3389/fmicb.2021.708480. eCollection 2021. Front Microbiol. 2021. PMID: 34335544 Free PMC article.
-
Transglycosylation of Stevioside by a Commercial β-Glucanase with Fungal Extracted β-Glucans as Donors.Waste Biomass Valorization. 2023 Jan 24:1-11. doi: 10.1007/s12649-023-02052-4. Online ahead of print. Waste Biomass Valorization. 2023. PMID: 36713934 Free PMC article.
References
-
- Roesijadi G, Jones SB, Snowden-Swan LJ, Zhu Y. 2010. Macroalgae as a biomass feedstock: a preliminary analysis. Publication PNNL-19944. Pacific Northwest National Laboratory, Richland, WA: http://www.pnl.gov/main/publications/external/technical_reports/PNNL-199....
-
- Anastasakis K, Ross AB, Jones JM. 2011. Pyrolysis behaviour of the main carbohydrates of brown macro-algae. Fuel 90:598–607. doi:10.1016/j.fuel.2010.09.023. - DOI
-
- Kadam SU, Tiwari BK, O'Donnell CP. 2015. Extraction, structure and biofunctional activities of laminarin from brown algae. Int J Food Sci Technol 50:24–31. doi:10.1111/ijfs.12692. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

