Energetics and Application of Heterotrophy in Acetogenic Bacteria
- PMID: 27208103
- PMCID: PMC4959221
- DOI: 10.1128/AEM.00882-16
Energetics and Application of Heterotrophy in Acetogenic Bacteria
Abstract
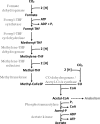
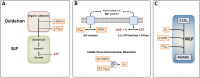
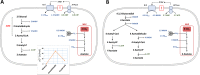
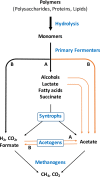
Acetogenic bacteria are a diverse group of strictly anaerobic bacteria that utilize the Wood-Ljungdahl pathway for CO2 fixation and energy conservation. These microorganisms play an important part in the global carbon cycle and are a key component of the anaerobic food web. Their most prominent metabolic feature is autotrophic growth with molecular hydrogen and carbon dioxide as the substrates. However, most members also show an outstanding metabolic flexibility for utilizing a vast variety of different substrates. In contrast to autotrophic growth, which is hardly competitive, metabolic flexibility is seen as a key ability of acetogens to compete in ecosystems and might explain the almost-ubiquitous distribution of acetogenic bacteria in anoxic environments. This review covers the latest findings with respect to the heterotrophic metabolism of acetogenic bacteria, including utilization of carbohydrates, lactate, and different alcohols, especially in the model acetogen Acetobacterium woodii Modularity of metabolism, a key concept of pathway design in synthetic biology, together with electron bifurcation, to overcome energetic barriers, appears to be the basis for the amazing substrate spectrum. At the same time, acetogens depend on only a relatively small number of enzymes to expand the substrate spectrum. We will discuss the energetic advantages of coupling CO2 reduction to fermentations that exploit otherwise-inaccessible substrates and the ecological advantages, as well as the biotechnological applications of the heterotrophic metabolism of acetogens.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures




Similar articles
-
Alanine, a Novel Growth Substrate for the Acetogenic Bacterium Acetobacterium woodii.Appl Environ Microbiol. 2018 Nov 15;84(23):e02023-18. doi: 10.1128/AEM.02023-18. Print 2018 Dec 1. Appl Environ Microbiol. 2018. PMID: 30242008 Free PMC article.
-
Comparative reaction engineering analysis of different acetogenic bacteria for gas fermentation.J Biotechnol. 2016 Jun 20;228:82-94. doi: 10.1016/j.jbiotec.2016.04.032. Epub 2016 Apr 20. J Biotechnol. 2016. PMID: 27107467
-
Nonacetogenic growth of the acetogen Acetobacterium woodii on 1,2-propanediol.J Bacteriol. 2015 Jan;197(2):382-91. doi: 10.1128/JB.02383-14. Epub 2014 Nov 10. J Bacteriol. 2015. PMID: 25384483 Free PMC article.
-
New Horizons in Acetogenic Conversion of One-Carbon Substrates and Biological Hydrogen Storage.Trends Biotechnol. 2019 Dec;37(12):1344-1354. doi: 10.1016/j.tibtech.2019.05.008. Epub 2019 Jun 27. Trends Biotechnol. 2019. PMID: 31257058 Review.
-
Analysis of the Core Genome and Pan-Genome of Autotrophic Acetogenic Bacteria.Front Microbiol. 2016 Sep 28;7:1531. doi: 10.3389/fmicb.2016.01531. eCollection 2016. Front Microbiol. 2016. PMID: 27733845 Free PMC article. Review.
Cited by
-
Formate-Dependent Acetogenic Utilization of Glucose by the Fecal Acetogen Clostridium bovifaecis.Appl Environ Microbiol. 2020 Nov 10;86(23):e01870-20. doi: 10.1128/AEM.01870-20. Print 2020 Nov 10. Appl Environ Microbiol. 2020. PMID: 32948524 Free PMC article.
-
Effect of Humin and Chemical Factors on CO2-Fixing Acetogenesis and Methanogenesis.Int J Environ Res Public Health. 2022 Feb 22;19(5):2546. doi: 10.3390/ijerph19052546. Int J Environ Res Public Health. 2022. PMID: 35270239 Free PMC article.
-
Evidence of competition between electrogens shaping electroactive microbial communities in microbial electrolysis cells.Front Microbiol. 2022 Dec 16;13:959211. doi: 10.3389/fmicb.2022.959211. eCollection 2022. Front Microbiol. 2022. PMID: 36590422 Free PMC article.
-
Energy conservation under extreme energy limitation: the role of cytochromes and quinones in acetogenic bacteria.Extremophiles. 2021 Nov;25(5-6):413-424. doi: 10.1007/s00792-021-01241-0. Epub 2021 Sep 4. Extremophiles. 2021. PMID: 34480656 Free PMC article. Review.
-
Metagenomic Exploration Uncovers Several Novel 'Candidatus' Species Involved in Acetate Metabolism in High-Ammonia Thermophilic Biogas Processes.Microb Biotechnol. 2025 Mar;18(3):e70133. doi: 10.1111/1751-7915.70133. Microb Biotechnol. 2025. PMID: 40126889 Free PMC article.
References
-
- Fischer F, Lieske R, Winzler K. 1932. Biologische Gasreaktionen. II. Über die Bildung von Essigsäure bei der biologischen Umsetzung von Kohlenoxyd und Kohlensäure zu Methan. Biochem Z 245:2–12.
-
- Wieringa KT. 1936. Over het verdwijnen van waterstof en koolzuur onder anaerobe voorwaarden. Antonie Van Leeuwenhoek 3:263–273.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

