Unusual Butane- and Pentanetriol-Based Tetraether Lipids in Methanomassiliicoccus luminyensis, a Representative of the Seventh Order of Methanogens
- PMID: 27208108
- PMCID: PMC4984300
- DOI: 10.1128/AEM.00772-16
Unusual Butane- and Pentanetriol-Based Tetraether Lipids in Methanomassiliicoccus luminyensis, a Representative of the Seventh Order of Methanogens
Abstract
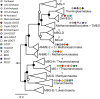
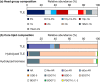
A new clade of archaea has recently been proposed to constitute the seventh methanogenic order, the Methanomassiliicoccales, which is related to the Thermoplasmatales and the uncultivated archaeal clades deep-sea hydrothermal vent Euryarchaeota group 2 and marine group II Euryarchaeota but only distantly related to other methanogens. In this study, we investigated the membrane lipid composition of Methanomassiliicoccus luminyensis, the sole cultured representative of this seventh order. The lipid inventory of M. luminyensis comprises a unique assemblage of novel lipids as well as lipids otherwise typical for thermophilic, methanogenic, or halophilic archaea. For instance, glycerol sesterpanyl-phytanyl diether core lipids found mainly in halophilic archaea were detected, and so were compounds bearing either heptose or methoxylated glycosidic head groups, neither of which have been reported so far for other archaea. The absence of quinones or methanophenazines is consistent with a biochemistry of methanogenesis different from that of the methanophenazine-containing methylotrophic methanogens. The most distinctive characteristic of the membrane lipid composition of M. luminyensis, however, is the presence of tetraether lipids in which one glycerol backbone is replaced by either butane- or pentanetriol, i.e., lipids recently discovered in marine sediments. Butanetriol dibiphytanyl glycerol tetraether (BDGT) constitutes the most abundant core lipid type (>50% relative abundance) in M. luminyensis We have thus identified a source for these unusual orphan lipids. The complementary analysis of diverse marine sediment samples showed that BDGTs are widespread in anoxic layers, suggesting an environmental significance of Methanomassiliicoccales and/or related BDGT producers beyond gastrointestinal tracts.
Importance: Cellular membranes of members of all three domains of life, Archaea, Bacteria, and Eukarya, are largely formed by lipids in which glycerol serves as backbone for the hydrophobic alkyl chains. Recently, however, archaeal tetraether lipids with either butanetriol or pentanetriol as a backbone were identified in marine sediments and attributed to uncultured sediment-dwelling archaea. Here we show that the butanetriol-based dibiphytanyl tetraethers constitute the major lipids in Methanomassiliicoccus luminyensis, currently the only isolate of the novel seventh order of methanogens. Given the absence of these lipids in a large set of archaeal isolates, these compounds may be diagnostic for the Methanomassiliicoccales and/or closely related archaea.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures




References
-
- Cicerone RJ, Oremland RS. 1988. Biogeochemical aspects of atmospheric methane. Global Biogeochem Cycles 2:299–327. doi:10.1029/GB002i004p00299. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

