Response of Vibrio cholerae to Low-Temperature Shifts: CspV Regulation of Type VI Secretion, Biofilm Formation, and Association with Zooplankton
- PMID: 27208110
- PMCID: PMC4959209
- DOI: 10.1128/AEM.00807-16
Response of Vibrio cholerae to Low-Temperature Shifts: CspV Regulation of Type VI Secretion, Biofilm Formation, and Association with Zooplankton
Abstract
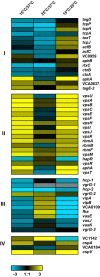
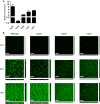
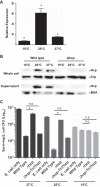
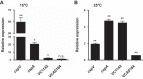
The ability to sense and adapt to temperature fluctuation is critical to the aquatic survival, transmission, and infectivity of Vibrio cholerae, the causative agent of the disease cholera. Little information is available on the physiological changes that occur when V. cholerae experiences temperature shifts. The genome-wide transcriptional profile of V. cholerae upon a shift in human body temperature (37°C) to lower temperatures, 15°C and 25°C, which mimic those found in the aquatic environment, was determined. Differentially expressed genes included those involved in the cold shock response, biofilm formation, type VI secretion, and virulence. Analysis of a mutant lacking the cold shock gene cspV, which was upregulated >50-fold upon a low-temperature shift, revealed that it regulates genes involved in biofilm formation and type VI secretion. CspV controls biofilm formation through modulation of the second messenger cyclic diguanylate and regulates type VI-mediated interspecies killing in a temperature-dependent manner. Furthermore, a strain lacking cspV had significant defects for attachment and type VI-mediated killing on the surface of the aquatic crustacean Daphnia magna Collectively, these studies reveal that cspV is a major regulator of the temperature downshift response and plays an important role in controlling cellular processes crucial to the infectious cycle of V. cholerae
Importance: Little is known about how human pathogens respond and adapt to ever-changing parameters of natural habitats outside the human host and how environmental adaptation alters dissemination. Vibrio cholerae, the causative agent of the severe diarrheal disease cholera, experiences fluctuations in temperature in its natural aquatic habitats and during the infection process. Furthermore, temperature is a critical environmental signal governing the occurrence of V. cholerae and cholera outbreaks. In this study, we showed that V. cholerae reprograms its transcriptome in response to fluctuations in temperature, which results in changes to biofilm formation and type VI secretion system activation. These processes in turn impact environmental survival and the virulence potential of this pathogen.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
The LonA Protease Regulates Biofilm Formation, Motility, Virulence, and the Type VI Secretion System in Vibrio cholerae.J Bacteriol. 2016 Jan 11;198(6):973-85. doi: 10.1128/JB.00741-15. J Bacteriol. 2016. PMID: 26755629 Free PMC article.
-
Cold shock response and major cold shock proteins of Vibrio cholerae.Appl Environ Microbiol. 2003 Nov;69(11):6361-9. doi: 10.1128/AEM.69.11.6361-6369.2003. Appl Environ Microbiol. 2003. PMID: 14602587 Free PMC article.
-
Competence-induced type VI secretion might foster intestinal colonization by Vibrio cholerae: Intestinal interbacterial killing by competence-induced V. cholerae.Bioessays. 2015 Nov;37(11):1163-8. doi: 10.1002/bies.201500101. Epub 2015 Sep 11. Bioessays. 2015. PMID: 26445388
-
Expression of Vibrio cholerae virulence genes in response to environmental signals.Curr Issues Intest Microbiol. 2002 Sep;3(2):29-38. Curr Issues Intest Microbiol. 2002. PMID: 12400636 Review.
-
H-NS: an overarching regulator of the Vibrio cholerae life cycle.Res Microbiol. 2017 Jan;168(1):16-25. doi: 10.1016/j.resmic.2016.07.007. Epub 2016 Aug 1. Res Microbiol. 2017. PMID: 27492955 Free PMC article. Review.
Cited by
-
The Xanthomonas citri pv. citri Type VI Secretion System is Induced During Epiphytic Colonization of Citrus.Curr Microbiol. 2019 Oct;76(10):1105-1111. doi: 10.1007/s00284-019-01735-3. Epub 2019 Jul 9. Curr Microbiol. 2019. PMID: 31289847
-
Insight into the resilience and susceptibility of marine bacteria to T6SS attack by Vibrio cholerae and Vibrio coralliilyticus.PLoS One. 2020 Jan 28;15(1):e0227864. doi: 10.1371/journal.pone.0227864. eCollection 2020. PLoS One. 2020. PMID: 31990915 Free PMC article.
-
Contribution of the cold shock protein CspA to virulence in Xanthomonas oryzae pv. oryzae.Mol Plant Pathol. 2019 Mar;20(3):382-391. doi: 10.1111/mpp.12763. Epub 2018 Nov 16. Mol Plant Pathol. 2019. PMID: 30372574 Free PMC article.
-
Killing by Type VI secretion drives genetic phase separation and correlates with increased cooperation.Nat Commun. 2017 Feb 6;8:14371. doi: 10.1038/ncomms14371. Nat Commun. 2017. PMID: 28165005 Free PMC article.
-
Killer prey: Ecology reverses bacterial predation.PLoS Biol. 2024 Jan 23;22(1):e3002454. doi: 10.1371/journal.pbio.3002454. eCollection 2024 Jan. PLoS Biol. 2024. PMID: 38261596 Free PMC article.
References
-
- Baffone W, Tarsi R, Pane L, Campana R, Repetto B, Mariottini GL, Pruzzo C. 2006. Detection of free-living and plankton-bound vibrios in coastal waters of the Adriatic Sea (Italy) and study of their pathogenicity-associated properties. Environ Microbiol 8:1299–1305. doi:10.1111/j.1462-2920.2006.01011.x. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

