A Light Switch Based on Protein S-Nitrosylation Fine-Tunes Photosynthetic Light Harvesting in Chlamydomonas
- PMID: 27208221
- PMCID: PMC4902583
- DOI: 10.1104/pp.15.01878
A Light Switch Based on Protein S-Nitrosylation Fine-Tunes Photosynthetic Light Harvesting in Chlamydomonas
Abstract
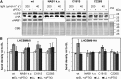
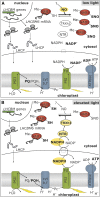
Photosynthetic eukaryotes are challenged by a fluctuating light supply, demanding for a modulated expression of nucleus-encoded light-harvesting proteins associated with photosystem II (LHCII) to adjust light-harvesting capacity to the prevailing light conditions. Here, we provide clear evidence for a regulatory circuit that controls cytosolic LHCII translation in response to light quantity changes. In the green unicellular alga Chlamydomonas reinhardtii, the cytosolic RNA-binding protein NAB1 represses translation of certain LHCII isoform mRNAs. Specific nitrosylation of Cys-226 decreases NAB1 activity and could be demonstrated in vitro and in vivo. The less active, nitrosylated form of NAB1 is found in cells acclimated to limiting light supply, which permits accumulation of light-harvesting proteins and efficient light capture. In contrast, elevated light supply causes its denitrosylation, thereby activating the repression of light-harvesting protein synthesis, which is needed to control excitation pressure at photosystem II. Denitrosylation of recombinant NAB1 is efficiently performed by the cytosolic thioredoxin system in vitro. To our knowledge, NAB1 is the first example of stimulus-induced denitrosylation in the context of photosynthetic acclimation. By identifying this novel redox cross-talk pathway between chloroplast and cytosol, we add a new key element required for drawing a precise blue print of the regulatory network of light harvesting.
© 2016 American Society of Plant Biologists. All Rights Reserved.
Figures





References
-
- Anderson JM, Chow WS, Park Y-I (1995) The grand design of photosynthesis: Acclimation of the photosynthetic apparatus to environmental cues. Photosynth Res 46: 129–139 - PubMed
-
- Astier J, Rasul S, Koen E, Manzoor H, Besson-Bard A, Lamotte O, Jeandroz S, Durner J, Lindermayr C, Wendehenne D (2011) S-nitrosylation: an emerging post-translational protein modification in plants. Plant Sci 181: 527–533 - PubMed
-
- Baudouin E. (2011) The language of nitric oxide signalling. Plant Biol (Stuttg) 13: 233–242 - PubMed
-
- Beckmann J, Lehr F, Finazzi G, Hankamer B, Posten C, Wobbe L, Kruse O (2009) Improvement of light to biomass conversion by de-regulation of light-harvesting protein translation in Chlamydomonas reinhardtii. J Biotechnol 142: 70–77 - PubMed
-
- Bedhomme M, Adamo M, Marchand CH, Couturier J, Rouhier N, Lemaire SD, Zaffagnini M, Trost P (2012) Glutathionylation of cytosolic glyceraldehyde-3-phosphate dehydrogenase from the model plant Arabidopsis thaliana is reversed by both glutaredoxins and thioredoxins in vitro. Biochem J 445: 337–347 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

