Loss of Mitochondrial Malate Dehydrogenase Activity Alters Seed Metabolism Impairing Seed Maturation and Post-Germination Growth in Arabidopsis
- PMID: 27208265
- PMCID: PMC4902577
- DOI: 10.1104/pp.16.01654
Loss of Mitochondrial Malate Dehydrogenase Activity Alters Seed Metabolism Impairing Seed Maturation and Post-Germination Growth in Arabidopsis
Abstract
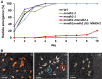
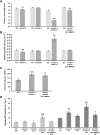
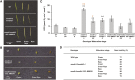
Mitochondrial malate dehydrogenase (mMDH; EC 1.1.1.37) has multiple roles; the most commonly described is its catalysis of the interconversion of malate and oxaloacetate in the tricarboxylic acid cycle. The roles of mMDH in Arabidopsis (Arabidopsis thaliana) seed development and germination were investigated in mMDH1 and mMDH2 double knockout plants. A significant proportion of mmdh1mmdh2 seeds were nonviable and developed only to torpedo-shaped embryos, indicative of arrested seed embryo growth during embryogenesis. The viable mmdh1mmdh2 seeds had an impaired maturation process that led to slow germination rates as well as retarded post-germination growth, shorter root length, and decreased root biomass. During seed development, mmdh1mmdh2 showed a paler green phenotype than the wild type and exhibited deficiencies in reserve accumulation and reduced final seed biomass. The respiration rate of mmdh1mmdh2 seeds was significantly elevated throughout their maturation, consistent with the previously reported higher respiration rate in mmdh1mmdh2 leaves. Mutant seeds showed a consistently higher content of free amino acids (branched-chain amino acids, alanine, serine, glycine, proline, and threonine), differences in sugar and sugar phosphate levels, and lower content of 2-oxoglutarate. Seed-aging assays showed that quiescent mmdh1mmdh2 seeds lost viability more than 3 times faster than wild-type seeds. Together, these data show the important role of mMDH in the earliest phases of the life cycle of Arabidopsis.
© 2016 American Society of Plant Biologists. All Rights Reserved.
Figures

Similar articles
-
Mitochondrial malate dehydrogenase lowers leaf respiration and alters photorespiration and plant growth in Arabidopsis.Plant Physiol. 2010 Nov;154(3):1143-57. doi: 10.1104/pp.110.161612. Epub 2010 Sep 27. Plant Physiol. 2010. PMID: 20876337 Free PMC article.
-
Fatty acid beta-oxidation in germinating Arabidopsis seeds is supported by peroxisomal hydroxypyruvate reductase when malate dehydrogenase is absent.Plant Mol Biol. 2010 Jan;72(1-2):101-9. doi: 10.1007/s11103-009-9554-2. Epub 2009 Oct 8. Plant Mol Biol. 2010. PMID: 19812894
-
The Arabidopsis E1 subunit of the 2-oxoglutarate dehydrogenase complex modulates plant growth and seed production.Plant Mol Biol. 2019 Sep;101(1-2):183-202. doi: 10.1007/s11103-019-00900-3. Epub 2019 Jul 8. Plant Mol Biol. 2019. PMID: 31286324
-
Non-coding and epigenetic mechanisms in the regulation of seed germination in Arabidopsis thaliana.J Exp Bot. 2025 Jun 17;76(9):2455-2467. doi: 10.1093/jxb/eraf051. J Exp Bot. 2025. PMID: 39918256 Review.
-
Perspectives on embryo maturation and seed quality in a global climate change scenario.J Exp Bot. 2024 Jul 23;75(14):4394-4399. doi: 10.1093/jxb/erae154. J Exp Bot. 2024. PMID: 38597771 Review.
Cited by
-
Proteome-wide analyses reveal diverse functions of protein acetylation and succinylation modifications in fast growing stolons of bermudagrass (Cynodon dactylon L.).BMC Plant Biol. 2022 Oct 27;22(1):503. doi: 10.1186/s12870-022-03885-2. BMC Plant Biol. 2022. PMID: 36289454 Free PMC article.
-
Mitochondrial redox systems as central hubs in plant metabolism and signaling.Plant Physiol. 2021 May 27;186(1):36-52. doi: 10.1093/plphys/kiab101. Plant Physiol. 2021. PMID: 33624829 Free PMC article. Review.
-
Genome-Wide Identification of AhMDHs and Analysis of Gene Expression under Manganese Toxicity Stress in Arachis hypogaea.Genes (Basel). 2023 Nov 21;14(12):2109. doi: 10.3390/genes14122109. Genes (Basel). 2023. PMID: 38136931 Free PMC article.
-
Seed comparative genomics in three coffee species identify desiccation tolerance mechanisms in intermediate seeds.J Exp Bot. 2020 Feb 19;71(4):1418-1433. doi: 10.1093/jxb/erz508. J Exp Bot. 2020. PMID: 31790120 Free PMC article.
-
Novel Cytonuclear Combinations Modify Arabidopsis thaliana Seed Physiology and Vigor.Front Plant Sci. 2019 Feb 5;10:32. doi: 10.3389/fpls.2019.00032. eCollection 2019. Front Plant Sci. 2019. PMID: 30804952 Free PMC article.
References
-
- Alhamdan AM, Alsadon AA, Khalil SO, Allah MA, Nagar ME, Ibrahim AA (2011) Influence of storage conditions on seed quality and longevity of four vegetable crops. Am Eur J Agric Environ Sci 11: 353–359
-
- Amable RA, Obendorf RL (1986) Soybean seed respiration during simulated preharvest deterioration. J Exp Bot 37: 1364–1375
-
- Araújo WL, Ishizaki K, Nunes-Nesi A, Larson TR, Tohge T, Krahnert I, Witt S, Obata T, Schauer N, Graham IA, et al. (2010) Identification of the 2-hydroxyglutarate and isovaleryl-CoA dehydrogenases as alternative electron donors linking lysine catabolism to the electron transport chain of Arabidopsis mitochondria. Plant Cell 22: 1549–1563 - PMC - PubMed
-
- Araújo WL, Nunes-Nesi A, Nikoloski Z, Sweetlove LJ, Fernie AR (2012) Metabolic control and regulation of the tricarboxylic acid cycle in photosynthetic and heterotrophic plant tissues. Plant Cell Environ 35: 1–21 - PubMed
-
- Araújo WL, Tohge T, Ishizaki K, Leaver CJ, Fernie AR (2011) Protein degradation: an alternative respiratory substrate for stressed plants. Trends Plant Sci 16: 489–498 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

