Distinguishing the Roles of Thylakoid Respiratory Terminal Oxidases in the Cyanobacterium Synechocystis sp. PCC 6803
- PMID: 27208274
- PMCID: PMC4902628
- DOI: 10.1104/pp.16.00479
Distinguishing the Roles of Thylakoid Respiratory Terminal Oxidases in the Cyanobacterium Synechocystis sp. PCC 6803
Abstract
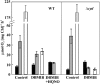
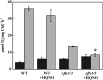
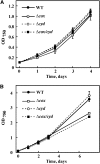
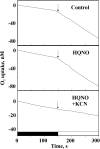
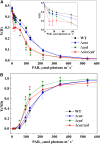
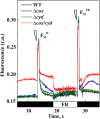
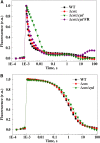
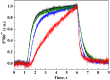
Various oxygen-utilizing electron sinks, including the soluble flavodiiron proteins (Flv1/3), and the membrane-localized respiratory terminal oxidases (RTOs), cytochrome c oxidase (Cox) and cytochrome bd quinol oxidase (Cyd), are present in the photosynthetic electron transfer chain of Synechocystis sp. PCC 6803. However, the role of individual RTOs and their relative importance compared with other electron sinks are poorly understood, particularly under light. Via membrane inlet mass spectrometry gas exchange, chlorophyll a fluorescence, P700 analysis, and inhibitor treatment of the wild type and various mutants deficient in RTOs, Flv1/3, and photosystem I, we investigated the contribution of these complexes to the alleviation of excess electrons in the photosynthetic chain. To our knowledge, for the first time, we demonstrated the activity of Cyd in oxygen uptake under light, although it was detected only upon inhibition of electron transfer at the cytochrome b6f site and in ∆flv1/3 under fluctuating light conditions, where linear electron transfer was drastically inhibited due to impaired photosystem I activity. Cox is mostly responsible for dark respiration and competes with P700 for electrons under high light. Only the ∆cox/cyd double mutant, but not single mutants, demonstrated a highly reduced plastoquinone pool in darkness and impaired gross oxygen evolution under light, indicating that thylakoid-based RTOs are able to compensate partially for each other. Thus, both electron sinks contribute to the alleviation of excess electrons under illumination: RTOs continue to function under light, operating on slower time ranges and on a limited scale, whereas Flv1/3 responds rapidly as a light-induced component and has greater capacity.
© 2016 American Society of Plant Biologists. All Rights Reserved.
Figures

Similar articles
-
Photosystem activity and state transitions of the photosynthetic apparatus in cyanobacterium Synechocystis PCC 6803 mutants with different redox state of the plastoquinone pool.Biochemistry (Mosc). 2015 Jan;80(1):50-60. doi: 10.1134/S000629791501006X. Biochemistry (Mosc). 2015. PMID: 25754039
-
Thylakoid terminal oxidases are essential for the cyanobacterium Synechocystis sp. PCC 6803 to survive rapidly changing light intensities.Plant Physiol. 2013 May;162(1):484-95. doi: 10.1104/pp.112.210260. Epub 2013 Mar 5. Plant Physiol. 2013. PMID: 23463783 Free PMC article.
-
Disruption of the ndhF1 gene affects Chl fluorescence through state transition in the Cyanobacterium Synechocystis sp. PCC 6803, resulting in apparent high efficiency of photosynthesis.Plant Cell Physiol. 2013 Jul;54(7):1164-71. doi: 10.1093/pcp/pct068. Epub 2013 May 2. Plant Cell Physiol. 2013. PMID: 23645628
-
Photoprotection of photosystems in fluctuating light intensities.J Exp Bot. 2015 May;66(9):2427-36. doi: 10.1093/jxb/eru463. Epub 2014 Dec 1. J Exp Bot. 2015. PMID: 25468932 Review.
-
The cytochrome b6f complex at the crossroad of photosynthetic electron transport pathways.Plant Physiol Biochem. 2014 Aug;81:163-83. doi: 10.1016/j.plaphy.2013.12.011. Epub 2013 Dec 27. Plant Physiol Biochem. 2014. PMID: 24485217 Review.
Cited by
-
Improved photosynthetic capacity and photosystem I oxidation via heterologous metabolism engineering in cyanobacteria.Proc Natl Acad Sci U S A. 2021 Mar 16;118(11):e2021523118. doi: 10.1073/pnas.2021523118. Proc Natl Acad Sci U S A. 2021. PMID: 33836593 Free PMC article.
-
Overexpression of plastid terminal oxidase in Synechocystis sp. PCC 6803 alters cellular redox state.Philos Trans R Soc Lond B Biol Sci. 2017 Sep 26;372(1730):20160379. doi: 10.1098/rstb.2016.0379. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28808098 Free PMC article.
-
Characterization of the wave phenomenon of flash-induced chlorophyll fluorescence in Chlamydomonas reinhardtii.Photosynth Res. 2022 May;152(2):235-244. doi: 10.1007/s11120-022-00900-3. Epub 2022 Feb 15. Photosynth Res. 2022. PMID: 35166999 Free PMC article.
-
6PPD-quinone affects the photosynthetic carbon fixation in cyanobacteria by extracting photosynthetic electrons.Innovation (Camb). 2024 Apr 26;5(4):100630. doi: 10.1016/j.xinn.2024.100630. eCollection 2024 Jul 1. Innovation (Camb). 2024. PMID: 38800352 Free PMC article.
-
Photosynthetic sea slugs induce protective changes to the light reactions of the chloroplasts they steal from algae.Elife. 2020 Oct 20;9:e57389. doi: 10.7554/eLife.57389. Elife. 2020. PMID: 33077025 Free PMC article.
References
-
- Abramson J, Riistama S, Larsson G, Jasaitis A, Svensson-Ek M, Laakkonen L, Puustinen A, Iwata S, Wikström M (2000) The structure of the ubiquinol oxidase from Escherichia coli and its ubiquinone binding site. Nat Struct Biol 7: 910–917 - PubMed
-
- Ardelean II, Peschek GA (2011) The site of respiratory electron transport in cyanobacteria and its implication for the photo-inhibition of respiration. In Peschek GA, Obinger C, Renger G, eds, Bioenergetic Processes of Cyanobacteria: From Evolutionary Singularity to Ecological Diversity. Springer, New York, pp 131–136
-
- Bailey S, Melis A, Mackey KR, Cardol P, Finazzi G, van Dijken G, Berg GM, Arrigo K, Shrager J, Grossman A (2008) Alternative photosynthetic electron flow to oxygen in marine Synechococcus. Biochim Biophys Acta 1777: 269–276 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

