A Single Amino-Acid Substitution in the Sodium Transporter HKT1 Associated with Plant Salt Tolerance
- PMID: 27208305
- PMCID: PMC4936583
- DOI: 10.1104/pp.16.00569
A Single Amino-Acid Substitution in the Sodium Transporter HKT1 Associated with Plant Salt Tolerance
Abstract
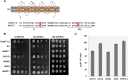
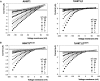
A crucial prerequisite for plant growth and survival is the maintenance of potassium uptake, especially when high sodium surrounds the root zone. The Arabidopsis HIGH-AFFINITY K(+) TRANSPORTER1 (HKT1), and its homologs in other salt-sensitive dicots, contributes to salinity tolerance by removing Na(+) from the transpiration stream. However, TsHKT1;2, one of three HKT1 copies in Thellungiella salsuginea, a halophytic Arabidopsis relative, acts as a K(+) transporter in the presence of Na(+) in yeast (Saccharomyces cerevisiae). Amino-acid sequence comparisons indicated differences between TsHKT1;2 and most other published HKT1 sequences with respect to an Asp residue (D207) in the second pore-loop domain. Two additional T salsuginea and most other HKT1 sequences contain Asn (n) in this position. Wild-type TsHKT1;2 and altered AtHKT1 (AtHKT1(N-D)) complemented K(+)-uptake deficiency of yeast cells. Mutant hkt1-1 plants complemented with both AtHKT1(N) (-) (D) and TsHKT1;2 showed higher tolerance to salt stress than lines complemented by the wild-type AtHKT1 Electrophysiological analysis in Xenopus laevis oocytes confirmed the functional properties of these transporters and the differential selectivity for Na(+) and K(+) based on the n/d variance in the pore region. This change also dictated inward-rectification for Na(+) transport. Thus, the introduction of Asp, replacing Asn, in HKT1-type transporters established altered cation selectivity and uptake dynamics. We describe one way, based on a single change in a crucial protein that enabled some crucifer species to acquire improved salt tolerance, which over evolutionary time may have resulted in further changes that ultimately facilitated colonization of saline habitats.
© 2016 American Society of Plant Biologists. All Rights Reserved.
Figures





References
-
- Ali Z, Park HC, Ali A, Oh DH, Aman R, Kropornicka A, Hong H, Choi W, Chung WS, Kim WY, Bressan RA, Bohnert HJ, et al. (2012) TsHKT1;2, a HKT1 homolog from the extremophile Arabidopsis relative Thellungiella salsuginea, shows K+ specificity in the presence of NaCl. Plant Physiol 158: 1463–1474 - PMC - PubMed
-
- Bañuelos MA, Haro R, Fraile-Escanciano A, Rodríguez-Navarro A (2008) Effects of polylinker uATGs on the function of grass HKT1 transporters expressed in yeast cells. Plant Cell Physiol 49: 1128–1132 - PubMed
-
- Batelli G, Oh DH, D’Urzo MP, Orsini F, Dassanayake M, Zhu JK, Bohnert HJ, Bressan RA, Maggio A (2014) Using Arabidopsis-related model species (ARMS): growth, genetic transformation, and comparative genomics. Methods Mol Biol 1062: 27–51 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

