Phase 3 Trial of Transplantation of Human Islets in Type 1 Diabetes Complicated by Severe Hypoglycemia
- PMID: 27208344
- PMCID: PMC5317236
- DOI: 10.2337/dc15-1988
Phase 3 Trial of Transplantation of Human Islets in Type 1 Diabetes Complicated by Severe Hypoglycemia
Abstract
Objective: Impaired awareness of hypoglycemia (IAH) and severe hypoglycemic events (SHEs) cause substantial morbidity and mortality in patients with type 1 diabetes (T1D). Current therapies are effective in preventing SHEs in 50-80% of patients with IAH and SHEs, leaving a substantial number of patients at risk. We evaluated the effectiveness and safety of a standardized human pancreatic islet product in subjects in whom IAH and SHEs persisted despite medical treatment.
Research design and methods: This multicenter, single-arm, phase 3 study of the investigational product purified human pancreatic islets (PHPI) was conducted at eight centers in North America. Forty-eight adults with T1D for >5 years, absent stimulated C-peptide, and documented IAH and SHEs despite expert care were enrolled. Each received immunosuppression and one or more transplants of PHPI, manufactured on-site under good manufacturing practice conditions using a common batch record and standardized lot release criteria and test methods. The primary end point was the achievement of HbA1c <7.0% (53 mmol/mol) at day 365 and freedom from SHEs from day 28 to day 365 after the first transplant.
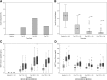
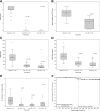
Results: The primary end point was successfully met by 87.5% of subjects at 1 year and by 71% at 2 years. The median HbA1c level was 5.6% (38 mmol/mol) at both 1 and 2 years. Hypoglycemia awareness was restored, with highly significant improvements in Clarke and HYPO scores (P > 0.0001). No study-related deaths or disabilities occurred. Five of the enrollees (10.4%) experienced bleeds requiring transfusions (corresponding to 5 of 75 procedures), and two enrollees (4.1%) had infections attributed to immunosuppression. Glomerular filtration rate decreased significantly on immunosuppression, and donor-specific antibodies developed in two patients.
Conclusions: Transplanted PHPI provided glycemic control, restoration of hypoglycemia awareness, and protection from SHEs in subjects with intractable IAH and SHEs. Safety events occurred related to the infusion procedure and immunosuppression, including bleeding and decreased renal function. Islet transplantation should be considered for patients with T1D and IAH in whom other, less invasive current treatments have been ineffective in preventing SHEs.
Trial registration: ClinicalTrials.gov NCT00434811.
© 2016 by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered.
Figures

Comment in
-
Diabetes: Islet transplantation for T1DM.Nat Rev Endocrinol. 2016 Jul;12(7):373. doi: 10.1038/nrendo.2016.68. Epub 2016 May 6. Nat Rev Endocrinol. 2016. PMID: 27150290 No abstract available.
-
Islet Transplantation for Hypoglycemia Unawareness/Severe Hypoglycemia: Caveat Emptor.Diabetes Care. 2016 Jul;39(7):1072-4. doi: 10.2337/dci16-0008. Diabetes Care. 2016. PMID: 27330121 No abstract available.
References
-
- Cryer PE. Glycemic goals in diabetes: trade-off between glycemic control and iatrogenic hypoglycemia. Diabetes 2014;63:2188–2195 - PubMed
-
- Geddes J, Schopman JE, Zammitt NN, Frier BM. Prevalence of impaired awareness of hypoglycaemia in adults with type 1 diabetes. Diabet Med 2008;25:501–504 - PubMed
-
- Weinstock RS, Xing D, Maahs DM, et al. .; T1D Exchange Clinic Network . Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D Exchange clinic registry. J Clin Endocrinol Metab 2013;98:3411–3419 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- M01 RR000400/RR/NCRR NIH HHS/United States
- N01 AI015416/AI/NIAID NIH HHS/United States
- M01 RR000040/RR/NCRR NIH HHS/United States
- U01 AI065192/AI/NIAID NIH HHS/United States
- UL1 RR025741/RR/NCRR NIH HHS/United States
- UL1 TR000114/TR/NCATS NIH HHS/United States
- U01 DK070460/DK/NIDDK NIH HHS/United States
- UL1 TR000003/TR/NCATS NIH HHS/United States
- U01 AI065193/AI/NIAID NIH HHS/United States
- UL1 TR000004/TR/NCATS NIH HHS/United States
- U01 DK070430/DK/NIDDK NIH HHS/United States
- U01 AI089316/AI/NIAID NIH HHS/United States
- UM1 AI109565/AI/NIAID NIH HHS/United States
- U01 AI089317/AI/NIAID NIH HHS/United States
- U01 DK085531/DK/NIDDK NIH HHS/United States
- U01 DK070431/DK/NIDDK NIH HHS/United States
- UL1 TR000150/TR/NCATS NIH HHS/United States
- U01 AI065191/AI/NIAID NIH HHS/United States
- UL1 TR000050/TR/NCATS NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- UL1 TR000460/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

