Clinical Effects of a Topically Applied Toll-like Receptor 9 Agonist in Active Moderate-to-Severe Ulcerative Colitis
- PMID: 27208386
- PMCID: PMC5091328
- DOI: 10.1093/ecco-jcc/jjw103
Clinical Effects of a Topically Applied Toll-like Receptor 9 Agonist in Active Moderate-to-Severe Ulcerative Colitis
Abstract
Background and aims: Toll-like receptors [TLRs] are potential drug targets for immunomodulation. We determined the safety and efficacy of the TLR-9 agonist DNA-based immunomodulatory sequence 0150 [DIMS0150] in ulcerative colitis [UC] patients refractory to standard therapy.
Methods: In this randomized, double-blind, placebo-controlled trial, 131 patients with moderate-to-severe active UC were randomized to receive two single doses of the oligonucleotide DIMS0150 [30 mg] or placebo administered topically during lower GI endoscopy at baseline and Week 4. The primary endpoint was clinical remission, defined as Clinical Activity Index [CAI] ≤4, at Week 12. Secondary endpoints included mucosal healing and symptomatic remission of key patient-reported outcomes [absence of blood in stool and weekly stool frequency <35].
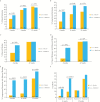
Results: There was no statistical significant difference between the groups in the induction of clinical remission at Week 12, with 44.4% in the DIMS0150 group vs. 46.5% in the placebo group. However, the proportion of patients who achieved symptomatic remission was 32.1% in the DIMS0150 group vs. 14.0% in the placebo group at Week 4 [p = 0.020], and 44.4% vs. 27.9% at Week 8 [p = 0.061]. More patients on DIMS0150 compared with those on placebo had mucosal healing [34.6% vs. 18.6%; p = 0.09] and histological improvement regarding the Geboes score [30.9% vs. 9.3%; p = 0.0073] at Week 4. Significantly more patients on DIMS0150 were in clinical remission with mucosal healing at Week 4: 21% vs. 4.7% in the placebo group [p = 0.02]. DIMS0150 was well tolerated, and no safety signals compared with placebo were evident.
Conclusions: Therapy with the topically applied TLR-9 agonist DIMS0150 is a promising and well-tolerated novel therapeutic option for treatment-refractory, chronic active UC patients, warranting further clinical trials.
Keywords: Toll-like receptors; therapy; ulcerative colitis.
© European Crohn’s and Colitis Organisation 2016.
Figures
References
-
- Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med 2011;365:1713–25. - PubMed
-
- Kornbluth A, Sachar DB. Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol 2010;105:501–23. - PubMed
-
- Dignass A, Lindsay JO, Sturm A, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis 2012;6:991–1030. - PubMed
-
- Feagan BG, Rutgeerts P, Sands BE, et al. ; GEMINI 1 Study Group Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699–710. - PubMed
-
- Ford AC, Sandborn WJ, Khan KJ, Hanauer SB, Talley NJ, Moayyedi P. Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol 2011;106:644–59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical