Collagen-collagen interactions mediated by plant-derived proanthocyanidins: A spectroscopic and atomic force microscopy study
- PMID: 27208639
- PMCID: PMC4983101
- DOI: 10.1016/j.actbio.2016.05.026
Collagen-collagen interactions mediated by plant-derived proanthocyanidins: A spectroscopic and atomic force microscopy study
Abstract
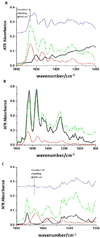
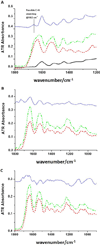
Collagen cross-linkings are determinant of biological tissue stability and function. Plant-derived proanthocyanidins (PACs) mimic different hierarchical levels of collagen cross-links by non-enzymatic interactions resulting in the enhancement to the biomechanics and biostability of collagen-rich tissues such as dentin. This study investigated the interaction of PACs from Vitis vinifera grape seed extract with type I collagen in solubilized form and in the demineralized dentin matrix (DDM) by fluorescence spectral analysis; collagen-collagen binding forces in presence of cross-linking solutions by atomic force microscopy (AFM); and spectroscopic analysis of the DDM using attenuated total reflectance Fourier transform-infrared spectroscopy (ATR-FTIR). Glutaraldehyde (GA) and carbodiimide hydrochloride (EDC) with known cross-linking mechanisms were selected for comparative analyses. Changes in fluorescence upon interaction of solubilized type I collagen with PACs, EDC and GA reflected pronounced modifications in collagen conformation. PACs also promoted stronger collagen-collagen fibrils interaction than EDC and GA. A new feature was observed using ATR-FTIR spectroscopic analysis in PACs-treated collagen and DDM. The findings suggest covalent interactions between collagen and PACs. The mechanisms of interaction between PACs-collagen hold attractive and promising tissue-tailored biomedical applications and the binding forces that potentially drive such interaction were characterized.
Statement of significance: Connective tissues such as skin, bone and dentin are mainly composed of type I collagen, which is cross-linked to promote tissue stability, strength and function. Novel therapies using substances that mimic cross-links have been proposed to promote repair of collagen-based-tissues. In dentistry, naturally occurring proanthocyanidins (PACs) have the potential to enhance dentin mechanical properties and reduce its enzymatic degradation, but their mechanisms of cross-linking are unclear. The present study investigated the specific interactions between PACs-type I collagen in purified and dentin collagen and compared to the well described cross-linking mechanisms promoted by synthetic chemical substances. Findings reveal that covalent-like bonds are induced by plant PACs in type I collagen as well as in complex dental native tissue, promoting strong collagen-collagen interactions.
Keywords: ATR-FTIR spectroscopy; Atomic force microscopy; Collagen cross-linking; Dentin; Proanthocyanidins.
Copyright © 2016 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Figures




References
-
- Rivera EM, Yamauchi M. Site comparisons of dentine collagen cross-links from extracted human teeth. Arch. Oral Biol. 1993;38:541–546. - PubMed
-
- Knott L, Bailey AJ. Collagen cross-links in mineralizing tissues: a review of their chemistry, function, and clinical relevance. Bone. 1998;22:181–187. - PubMed
-
- Bedran-Russo AKB, Pashley DH, Agee K, Drummond JL, Miescke KJ. Changes in stiffness of demineralized dentin following application of collagen crosslinkers. J. Biomed. Mater. Res. B Appl. Biomater. 2008;86:330–334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

