New β-Lactamase Inhibitors in the Clinic
- PMID: 27208767
- PMCID: PMC4980828
- DOI: 10.1016/j.idc.2016.02.007
New β-Lactamase Inhibitors in the Clinic
Abstract
Given the serious medical burden of β-lactamases, many approaches are being used identify candidate agents for β-lactamase inhibition. Here, we review two β-lactam-β-lactamase inhibitor (BL-BLI) combinations, ceftolozane-tazobactam and ceftazidime-avibactam that recently entered the clinic. In addition, we focus on BL-BLI combinations in preclinical development that have demonstrated activity in clinical isolates via susceptibility testing and/or in in vivo models of infection. We highlight only the BLIs that are able to reduce the Clinical Laboratory Standards Institute (CLSI) breakpoints for the BL partner into the susceptible range. Our analysis includes the primary literature, meeting abstracts, as well as the patent literature.
Keywords: Boronic acids; Carbapenems; Diazabicyclooctanones; Inhibitor; Metallo-beta-lactamases; Monobactams; Sulfones; β-Lactamases.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
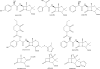
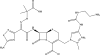
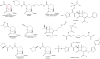
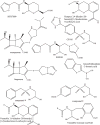
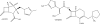
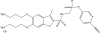
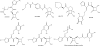
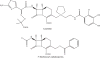
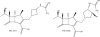
References
-
- Clinical and Laboratory Standards Institute. Twenty-fifth Informational supplement M100-S25. Wayne (PA): CLSI; 2015. Performance standards for antimicrobial susceptibility testing.
-
- Takeda S, Ishii Y, Hatano K, et al. Stability of FR264205 against AmpC β-lactamase of Pseudomonas aeruginosa. Int J Antimicrob Agents. 2007;30:443–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

