Late Ebola virus relapse causing meningoencephalitis: a case report
- PMID: 27209148
- PMCID: PMC4967715
- DOI: 10.1016/S0140-6736(16)30386-5
Late Ebola virus relapse causing meningoencephalitis: a case report
Abstract
Background: There are thousands of survivors of the 2014 Ebola outbreak in west Africa. Ebola virus can persist in survivors for months in immune-privileged sites; however, viral relapse causing life-threatening and potentially transmissible disease has not been described. We report a case of late relapse in a patient who had been treated for severe Ebola virus disease with high viral load (peak cycle threshold value 13.2).
Methods: A 39-year-old female nurse from Scotland, who had assisted the humanitarian effort in Sierra Leone, had received intensive supportive treatment and experimental antiviral therapies, and had been discharged with undetectable Ebola virus RNA in peripheral blood. The patient was readmitted to hospital 9 months after discharge with symptoms of acute meningitis, and was found to have Ebola virus in cerebrospinal fluid (CSF). She was treated with supportive therapy and experimental antiviral drug GS-5734 (Gilead Sciences, San Francisco, Foster City, CA, USA). We monitored Ebola virus RNA in CSF and plasma, and sequenced the viral genome using an unbiased metagenomic approach.
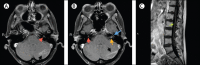
Findings: On admission, reverse transcriptase PCR identified Ebola virus RNA at a higher level in CSF (cycle threshold value 23.7) than plasma (31.3); infectious virus was only recovered from CSF. The patient developed progressive meningoencephalitis with cranial neuropathies and radiculopathy. Clinical recovery was associated with addition of high-dose corticosteroids during GS-5734 treatment. CSF Ebola virus RNA slowly declined and was undetectable following 14 days of treatment with GS-5734. Sequencing of plasma and CSF viral genome revealed only two non-coding changes compared with the original infecting virus.
Interpretation: Our report shows that previously unanticipated, late, severe relapses of Ebola virus can occur, in this case in the CNS. This finding fundamentally redefines what is known about the natural history of Ebola virus infection. Vigilance should be maintained in the thousands of Ebola survivors for cases of relapsed infection. The potential for these cases to initiate new transmission chains is a serious public health concern.
Funding: Royal Free London NHS Foundation Trust.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
Pauline Cafferkey: dedicated nurse and reluctant Ebola hero.Lancet. 2016 Jul 30;388(10043):455. doi: 10.1016/S0140-6736(16)30369-5. Epub 2016 May 18. Lancet. 2016. PMID: 27209147 No abstract available.
-
Defective interfering genomes and Ebola virus persistence.Lancet. 2016 Aug 13;388(10045):659-60. doi: 10.1016/S0140-6736(16)31272-7. Lancet. 2016. PMID: 27533436 No abstract available.
References
-
- WHO Ebola situation report. 20 January 2016. http://apps.who.int/ebola/current-situation/ebola-situation-report-20-ja... (accessed Jan 26, 2016).
-
- Qui X, Audet J, Wong G, et al. Successful treatment of Ebola virus-infected cynomolgus macaques with monoclonal antibodies. Sci Transl Med. 2012;4:138ra81. - PubMed
-
- Wilson AJ, Madox V, Rattenbury S, et al. Thromboelastography in the management of coagulopathy associated with Ebola virus disease. Clin Infect Dis. 2015;62:610–612. - PubMed
-
- Warren T, Jordan R, Lo M, et al. Once-daily treatment with GS-5734 initiated three days post viral challenge protects rhesus monkeys against lethal Ebola virus disease (EVD). ID Week; San Diego, CA, USA; Oct 7–11, 2015. LB-2 (abstr).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

