Potent social synchronization can override photic entrainment of circadian rhythms
- PMID: 27210069
- PMCID: PMC4879263
- DOI: 10.1038/ncomms11662
Potent social synchronization can override photic entrainment of circadian rhythms
Abstract
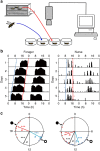
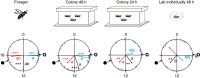
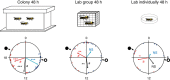
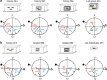
Circadian rhythms in behaviour and physiology are important for animal health and survival. Studies with individually isolated animals in the laboratory have consistently emphasized the dominant role of light for the entrainment of circadian rhythms to relevant environmental cycles. Although in nature interactions with conspecifics are functionally significant, social signals are typically not considered important time-givers for the animal circadian clock. Our results challenge this view. By studying honeybees in an ecologically relevant context and using a massive data set, we demonstrate that social entrainment can be potent, may act without direct contact with other individuals and does not rely on gating the exposure to light. We show for the first time that social time cues stably entrain the clock, even in animals experiencing conflicting photic and social environmental cycles. These findings add to the growing appreciation for the importance of studying circadian rhythms in ecologically relevant contexts.
Figures

Similar articles
-
Social synchronization of circadian rhythms with a focus on honeybees.Philos Trans R Soc Lond B Biol Sci. 2021 Oct 11;376(1835):20200342. doi: 10.1098/rstb.2020.0342. Epub 2021 Aug 23. Philos Trans R Soc Lond B Biol Sci. 2021. PMID: 34420390 Free PMC article.
-
Colony Volatiles and Substrate-borne Vibrations Entrain Circadian Rhythms and Are Potential Cues Mediating Social Synchronization in Honey Bee Colonies.J Biol Rhythms. 2020 Jun;35(3):246-256. doi: 10.1177/0748730420913362. Epub 2020 Apr 15. J Biol Rhythms. 2020. PMID: 32295458
-
Social influences on mammalian circadian rhythms: animal and human studies.Biol Rev Camb Philos Soc. 2004 Aug;79(3):533-56. doi: 10.1017/s1464793103006353. Biol Rev Camb Philos Soc. 2004. PMID: 15366762 Review.
-
The social clock of the honeybee.J Biol Rhythms. 2010 Oct;25(5):307-17. doi: 10.1177/0748730410380149. J Biol Rhythms. 2010. PMID: 20876811 Review.
-
The colony environment, but not direct contact with conspecifics, influences the development of circadian rhythms in honey bees.J Biol Rhythms. 2012 Jun;27(3):217-25. doi: 10.1177/0748730412440851. J Biol Rhythms. 2012. PMID: 22653890
Cited by
-
Social information as an entrainment cue for the circadian clock.Genet Mol Biol. 2024 Jul 22;47Suppl 1(Suppl 1):e20240008. doi: 10.1590/1678-4685-GMB-2024-0008. eCollection 2024. Genet Mol Biol. 2024. PMID: 39037375 Free PMC article.
-
Variation in chronotype is associated with migratory timing in a songbird.Biol Lett. 2019 Aug 30;15(8):20190453. doi: 10.1098/rsbl.2019.0453. Epub 2019 Aug 28. Biol Lett. 2019. PMID: 31455169 Free PMC article.
-
Shedding light on the circadian clock of the threespine stickleback.J Exp Biol. 2021 Dec 15;224(24):jeb242970. doi: 10.1242/jeb.242970. Epub 2021 Dec 17. J Exp Biol. 2021. PMID: 34854903 Free PMC article.
-
Clocks in the Wild: Entrainment to Natural Light.Front Physiol. 2020 Apr 2;11:272. doi: 10.3389/fphys.2020.00272. eCollection 2020. Front Physiol. 2020. PMID: 32300307 Free PMC article.
-
Pigment-dispersing factor is present in circadian clock neurons of pea aphids and may mediate photoperiodic signalling to insulin-producing cells.Open Biol. 2023 Jun;13(6):230090. doi: 10.1098/rsob.230090. Epub 2023 Jun 28. Open Biol. 2023. PMID: 37369351 Free PMC article.
References
-
- Espinasa L. & Jeffery W. R. Conservation of retinal circadian rhythms during cavefish eye degeneration. Evol. Dev. 8, 16–22 (2006). - PubMed
-
- Koilraj A. J., Sharma V. K., Marimuthu G. & Chandrashekaran M. K. Presence of circadian rhythms in the locomotor activity of a cave-dwelling millipede Glyphiulus cavernicolus sulu (Cambalidae, Spirostreptida). Chronobiol. Int. 17, 757–765 (2000). - PubMed
-
- Reichle D. E., Palmer J. D. & Park O. Persistent rhythmic locomotor activity in cave cricket hadenoecus subterraneus and its ecological significance. Am. Midl. Nat. 74, 57–66 (1965).
-
- Pittendrigh C. S. Temporal organization—reflections of a Darwinian clock-watcher. Annu. Rev. Physiol. 55, 16–54 (1993). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

