Size and targeting to PECAM vs ICAM control endothelial delivery, internalization and protective effect of multimolecular SOD conjugates
- PMID: 27210108
- PMCID: PMC4916012
- DOI: 10.1016/j.jconrel.2016.05.040
Size and targeting to PECAM vs ICAM control endothelial delivery, internalization and protective effect of multimolecular SOD conjugates
Abstract
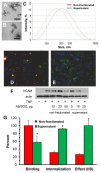
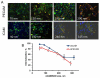
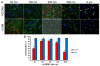
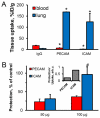
Controlled endothelial delivery of SOD may alleviate abnormal local surplus of superoxide involved in ischemia-reperfusion, inflammation and other disease conditions. Targeting SOD to endothelial surface vs. intracellular compartments is desirable to prevent pathological effects of external vs. endogenous superoxide, respectively. Thus, SOD conjugated with antibodies to cell adhesion molecule PECAM (Ab/SOD) inhibits pro-inflammatory signaling mediated by endogenous superoxide produced in the endothelial endosomes in response to cytokines. Here we defined control of surface vs. endosomal delivery and effect of Ab/SOD, focusing on conjugate size and targeting to PECAM vs. ICAM. Ab/SOD enlargement from about 100 to 300nm enhanced amount of cell-bound SOD and protection against extracellular superoxide. In contrast, enlargement inhibited endocytosis of Ab/SOD and diminished mitigation of inflammatory signaling of endothelial superoxide. In addition to size, shape is important: endocytosis of antibody-coated spheres was more effective than that of polymorphous antibody conjugates. Further, targeting to ICAM provides higher endocytic efficacy than targeting to PECAM. ICAM-targeted Ab/SOD more effectively mitigated inflammatory signaling by intracellular superoxide in vitro and in animal models, although total uptake was inferior to that of PECAM-targeted Ab/SOD. Therefore, both geometry and targeting features of Ab/SOD conjugates control delivery to cell surface vs. endosomes for optimal protection against extracellular vs. endosomal oxidative stress, respectively.
Keywords: Cell adhesion molecules; Endocytosis; Endothelial cells; Intracellular delivery; Vascular immunotargeting.
Copyright © 2016 Elsevier B.V. All rights reserved.
Figures




References
-
- den Hengst WA, Gielis JF, Lin JY, Van Schil PE, De Windt LJ, Moens AL. Lung ischemia-reperfusion injury: a molecular and clinical view on a complex pathophysiological process. Am J Physiol Heart Circ Physiol. 2010;299(5):H1283–1299. - PubMed
-
- Thomas SR, Witting PK, Drummond GR. Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2008;10(10):1713–1765. - PubMed
-
- Kowalski PS, Kuninty PR, Bijlsma KT, Stuart MC, Leus NG, Ruiters MH, Molema G, Kamps JA. SAINT-liposome-polycation particles, a new carrier for improved delivery of siRNAs to inflamed endothelial cells. Eur J Pharm Biopharm. 2015;89:40–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

