OCT-4 expression is essential for the segregation of trophectoderm lineages in porcine preimplantation embryos
- PMID: 27210587
- PMCID: PMC5004796
- DOI: 10.1262/jrd.2016-040
OCT-4 expression is essential for the segregation of trophectoderm lineages in porcine preimplantation embryos
Abstract
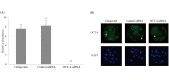
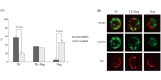
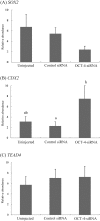
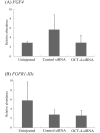
Oct-4, a member of the POU family of transcription factors, is a key factor that regulates the segregation of the inner cell mass (ICM) and the trophectoderm (TE) during the transition from morula to blastocyst in mice. However, little is known about its role in porcine early embryogenesis. To determine the function of OCT-4 in the ICM and TE segregation of porcine embryos, we studied the developmental morphology of porcine embryos using RNA interference technology. Our experiments demonstrated that when 1-cell stage embryos were co-injected with the small interfering RNA (siRNA)for targeted knockdown of OCT-4 (OCT-4-siRNA) and tetramethylrhodamine isothiocyanate (TRITC)-dextran conjugate (Dx), they failed to form blastocysts. Therefore, in this study, we constructed chimeric embryos comprising blastomeres that either expressed OCT-4 normally or showed downregulated OCT-4 expression by co-injection of OCT-4-siRNA and Dx into one blastomere in 2- to 4-cell stage embryos. In control embryos, which were co-injected with control siRNA and Dx, Dx-positive cells contributed to the TE lineage in almost all the blastocysts examined. In contrast, Dx-positive cells derived from a blastomere co-injected with OCT-4-siRNA and Dx were degenerated in almost half the blastocysts. This was probably due to the inability of these cells to differentiate into the TE lineage. Real-time RT-PCR analysis revealed no difference in the levels of SOX2, TEAD4, FGF4 and FGFR1-IIIc, all of which are known to be regulated by OCT-4, between the OCT-4-siRNA-injected morulae and the control ones. However, the level of CDX2, a molecule specifically expressed in the TE lineage, was significantly higher in the former than in the latter. Our results indicate that continuous expression of OCT-4 in blastomeres is essential for TE formation of porcine embryos.
Figures
References
-
- Yeom YI, Fuhrmann G, Ovitt CE, Brehm A, Ohbo K, Gross M, Hübner K, Schöler HR. Germline regulatory element of Oct-4 specific for the totipotent cycle of embryonal cells. Development 1996; 122: 881–894. - PubMed
-
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Schöler H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 1998; 95: 379–391. - PubMed
-
- Kirchhof N, Carnwath JW, Lemme E, Anastassiadis K, Schöler H, Niemann H. Expression pattern of Oct-4 in preimplantation embryos of different species. Biol Reprod 2000; 63: 1698–1705. - PubMed
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126: 663–676. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

