VEGF-D-enhanced lymph node metastasis of ovarian cancer is reversed by vesicular stomatitis virus matrix protein
- PMID: 27211072
- PMCID: PMC4902071
- DOI: 10.3892/ijo.2016.3527
VEGF-D-enhanced lymph node metastasis of ovarian cancer is reversed by vesicular stomatitis virus matrix protein
Abstract
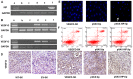
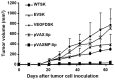
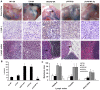
Lymphatic metastasis is a poor prognostic factor in ovarian cancer, which correlates to the majority of cancer deaths. Matrix protein (MP) of vesicular stomatitis virus (VSV) exhibits potent antitumor and antiangiogenic activities through inducing apoptosis and inhibiting angiogenesis. In this study, the antitumor and antimetastatic effects of MP were further investigated. Wild-type SKOV3 (WT-SK) cells were successfully transfected with empty vector pcDNA3.1 plasmid, or pcDNA3.1-VEGF-D recombinant plasmid to construct cell lines named EV-SK, and VEGFD-SK, respectively. Inhibition of VEGFD-SK cell migration and invasion was detected by Transwell and wound healing assay. Then, lymphogenous metastatic model of ovarian cancer was established by injecting VEGFD-SK cells subcutaneously into the left hindlimb claw pad of nude mice. The inducted apoptotic effect of MP on VEGFD-SK cells were assessed by flow analysis and Hoechst-33258 staining, respectively, in vitro. The in vivo antitumor and antiangiogenic activities of MP gene were evaluated with lymphogenous metastatic model of ovarian cancer. Tumor volume and lymphatic metastasis rates were measured. Lymphatic vessels were delineated using Evan's blue and LYVE-1 staining. Expression of VEGF-D and MMP-2 were evaluated by immunostaining. Apoptosis of tumor cells was analyzed by Hoechst-33258 staining. Mice bearing VEGFD-SK tumor cells displayed more rapid tumorigenesis, higher lymphogenous metastatic tendency and increased lymphatic vessel density compared with the mice bearing WT-SK or EV-SK cells. However, VEGF-D-enhanced metastasis was evidently reversed by MP. MP significantly reduced the invasion of VEGFD-SK cells, tumor volume, lymphatic metastasis rates and lymphatic vessel density compared with control groups (P<0.05), accompanied with down-expression of VEGF-D and MMP-2 and increased apoptosis. Our data indicate that MP has strong antitumor and antimetastatic abilities, and it may be a promising therapeutic strategy against the lymphatic metastasis of human ovarian cancer.
Figures



Similar articles
-
The antitumor and antimetastatic effects of N-trimethyl chitosan-encapsulated camptothecin on ovarian cancer with minimal side effects.Oncol Rep. 2010 Oct;24(4):941-8. doi: 10.3892/or.2010.941. Oncol Rep. 2010. PMID: 20811674
-
Efficient inhibition of intraperitoneal human ovarian cancer growth and prolonged survival by gene transfer of vesicular stomatitis virus matrix protein in nude mice.Gynecol Oncol. 2007 Mar;104(3):540-6. doi: 10.1016/j.ygyno.2006.09.022. Epub 2006 Nov 16. Gynecol Oncol. 2007. PMID: 17112567
-
VEGF-D-induced draining lymphatic enlargement and tumor lymphangiogenesis promote lymph node metastasis in a xenograft model of ovarian carcinoma.Reprod Biol Endocrinol. 2014 Feb 6;12:14. doi: 10.1186/1477-7827-12-14. Reprod Biol Endocrinol. 2014. PMID: 24502459 Free PMC article.
-
Molecular control of lymphatic metastasis.Ann N Y Acad Sci. 2008;1131:225-34. doi: 10.1196/annals.1413.020. Ann N Y Acad Sci. 2008. PMID: 18519975 Review.
-
Tumor angiogenesis and molecular target therapy in ovarian carcinomas.Hum Cell. 2005 Mar;18(1):1-16. doi: 10.1111/j.1749-0774.2005.tb00052.x. Hum Cell. 2005. PMID: 16130895 Review.
Cited by
-
Cancer-Specific Activation of the Vesicular Stomatitis Virus Matrix by Survivin Promoter in Breast Cancer Cells.Mol Biotechnol. 2025 Jan 17. doi: 10.1007/s12033-024-01359-4. Online ahead of print. Mol Biotechnol. 2025. PMID: 39820852
-
The Propagation and Quantification of Two Emerging Oncolytic Viruses: Vesicular Stomatitis (VSV) and Zika (ZIKV).Methods Mol Biol. 2020;2097:253-263. doi: 10.1007/978-1-0716-0203-4_16. Methods Mol Biol. 2020. PMID: 31776931 Free PMC article.
-
Targeting Multiple Signaling Pathways in Cancer: The Rutin Therapeutic Approach.Cancers (Basel). 2020 Aug 14;12(8):2276. doi: 10.3390/cancers12082276. Cancers (Basel). 2020. PMID: 32823876 Free PMC article. Review.
-
Oncotargeting by Vesicular Stomatitis Virus (VSV): Advances in Cancer Therapy.Viruses. 2018 Feb 23;10(2):90. doi: 10.3390/v10020090. Viruses. 2018. PMID: 29473868 Free PMC article. Review.
-
FTO/IGF2BP2-mediated N6 methyladenosine modification in invasion and metastasis of thyroid carcinoma via CDH12.Cell Death Dis. 2024 Oct 8;15(10):733. doi: 10.1038/s41419-024-07097-4. Cell Death Dis. 2024. PMID: 39379360 Free PMC article.
References
-
- Edwards BK, Brown ML, Wingo PA, Howe HL, Ward E, Ries LA, Schrag D, Jamison PM, Jemal A, Wu XC, et al. Annual report to the nation on the status of cancer, 1975–2002 featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97:1407–1427. doi: 10.1093/jnci/dji289. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

