Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis
- PMID: 27211272
- PMCID: PMC5399157
- DOI: 10.1038/leu.2016.148
Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis
Erratum in
-
Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis.Leukemia. 2017 Mar;31(3):775. doi: 10.1038/leu.2016.323. Leukemia. 2017. PMID: 28248313 Free PMC article. No abstract available.
Abstract
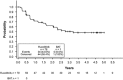
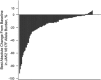
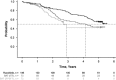
Ruxolitinib is a Janus kinase (JAK) (JAK1/JAK2) inhibitor that has demonstrated superiority over placebo and best available therapy (BAT) in the Controlled Myelofibrosis Study with Oral JAK Inhibitor Treatment (COMFORT) studies. COMFORT-II was a randomized (2:1), open-label phase 3 study in patients with myelofibrosis; patients randomized to BAT could crossover to ruxolitinib upon protocol-defined disease progression or after the primary end point, confounding long-term comparisons. At week 48, 28% (41/146) of patients randomized to ruxolitinib achieved ⩾35% decrease in spleen volume (primary end point) compared with no patients on BAT (P<0.001). Among the 78 patients (53.4%) in the ruxolitinib arm who achieved ⩾35% reductions in spleen volume at any time, the probability of maintaining response was 0.48 (95% confidence interval (CI), 0.35-0.60) at 5 years (median, 3.2 years). Median overall survival was not reached in the ruxolitinib arm and was 4.1 years in the BAT arm. There was a 33% reduction in risk of death with ruxolitinib compared with BAT by intent-to-treat analysis (hazard ratio (HR)=0.67; 95% CI, 0.44-1.02; P=0.06); the crossover-corrected HR was 0.44 (95% CI, 0.18-1.04; P=0.06). There was no unexpected increased incidence of adverse events with longer exposure. This final analysis showed that spleen volume reductions with ruxolitinib were maintained with continued therapy and may be associated with survival benefits.
Conflict of interest statement
CNH has received research support from Novartis, Cell Therapeutics, Gilead and Baxaltra through the institution; has received personal fees from Novartis, Shire, Gilead and Baxaltra; and has received grant and non-financial support from Novartis outside the submitted work. AMV has received grant and personal fees from Novartis during the conduct of the study. J-JK has received travel grant, and research funding paid to the institution from Novartis; has acted as a consultant to Novartis and Incyte. HKA-A has received research funding from Novartis and Celgene; acted as a consultant to Novartis; and has received honoraria from Novartis and Celgene. HG has received honoraria from Novartis, Celgene and AOP Orphan Pharmaceuticals; and has two licensed patents: EP 13.18.6939.8 and 13.18.4632.1. FC has received personal fees from Novartis, CTI-Baxter and Sanofi. MMJ and KS are employees of Incyte. MM and VS are employees of and own stock in Novartis. PG is an employee of Novartis.
Figures

References
-
- Vannucchi AM. Management of myelofibrosis. Hematol Am Soc Hematol Educ Program 2011; 2011: 222–230. - PubMed
-
- Vainchenker W, Delhommeau F, Constantinescu SN, Bernard OA. New mutations and pathogenesis of myeloproliferative neoplasms. Blood 2011; 188: 1723–1735. - PubMed
-
- Cervantes F, Dupriez B, Pereira A, PassamontiF, Reilly JT, Morra E et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood 2009; 113: 2895–2901. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

