The Role of Amyloid-β Oligomers in Toxicity, Propagation, and Immunotherapy
- PMID: 27211547
- PMCID: PMC4856795
- DOI: 10.1016/j.ebiom.2016.03.035
The Role of Amyloid-β Oligomers in Toxicity, Propagation, and Immunotherapy
Abstract
The incidence of Alzheimer's disease (AD) is growing every day and finding an effective treatment is becoming more vital. Amyloid-β (Aβ) has been the focus of research for several decades. The recent shift in the Aβ cascade hypothesis from all Aβ to small soluble oligomeric intermediates is directing the search for therapeutics towards the toxic mediators of the disease. Targeting the most toxic oligomers may prove to be an effective treatment by preventing their spread. Specific targeting of oligomers has been shown to protect cognition in rodent models. Additionally, the heterogeneity of research on Aβ oligomers may seem contradictory until size and conformation are taken into account. In this review, we will discuss Aβ oligomers and their toxicity in relation to size and conformation as well as their influence on inflammation and the potential of Aβ oligomer immunotherapy.
Keywords: Amyloid-β; Immunotherapy; Inflammation; Oligomers; Size; Toxicity.
Copyright © 2016 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

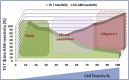
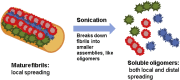
References
-
- Ahmed Z., Cooper J., Murray T., Garn K., Mcnaughton E., Clarke H., Parhizkar S., Ward M., Cavallini A., Jackson S., Bose S., Clavaguera F., Tolnay M., Lavenir I., Goedert M., Hutton M., O'neill M. A novel in vivo model of tau propagation with rapid and progressive neurofibrillary tangle pathology: the pattern of spread is determined by connectivity, not proximity. Acta Neuropathol. 2014;127:667–683. - PMC - PubMed
-
- Alzheimer's A. Alzheimer's disease facts and figures. Alzheimers Dement. 2015;11:332–384. - PubMed
-
- Bertram L., Lill C.M., Tanzi R.E. The genetics of Alzheimer disease: back to the future. Neuron. 2010;68:270–281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

