UFMylation: A Unique & Fashionable Modification for Life
- PMID: 27212118
- PMCID: PMC4936604
- DOI: 10.1016/j.gpb.2016.04.001
UFMylation: A Unique & Fashionable Modification for Life
Abstract
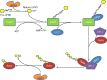
Ubiquitin-fold modifier 1 (UFM1) is one of the newly-identified ubiquitin-like proteins. Similar to ubiquitin, UFM1 is conjugated to its target proteins by a three-step enzymatic reaction. The UFM1-activating enzyme, ubiquitin-like modifier-activating enzyme 5 (UBA5), serves as the E1 to activate UFM1; UFM1-conjugating enzyme 1 (UFC1) acts as the E2 to transfer the activated UFM1 to the active site of the E2; and the UFM1-specific ligase 1 (UFL1) acts as the E3 to recognize its substrate, transfer, and ligate the UFM1 from E2 to the substrate. This process is called ufmylation. UFM1 chains can be cleaved from its target proteins by UFM1-specific proteases (UfSPs), suggesting that the ufmylation modification is reversible. UFM1 cascade is conserved among nearly all of the eukaryotic organisms, but not in yeast, and associated with several cellular activities including the endoplasmic reticulum stress response and hematopoiesis. Furthermore, the UFM1 cascade is closely related to a series of human diseases. In this review, we summarize the molecular details of this reversible modification process, the recent progress of its functional studies, as well as its implication in tumorigenesis and potential therapeutic targets for cancer.
Keywords: Cancer; Endoplasmic reticulum stress; Post-translation modification; Ubiquitin-fold modifier 1; Ubiquitin-like proteins; Ufmylation.
Copyright © 2016 The Authors. Production and hosting by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Hsu Y.C., Hsieh Y.H., Liao C.C., Chong L.W., Lee C.Y., Yu Y.L. Targeting post-translational modifications of histones for cancer therapy. Cell Mol Biol (Noisy-le-grand) 2015;61:69–84. - PubMed
-
- Hitosugi T., Chen J. Post-translational modifications and the Warburg effect. Oncogene. 2014;33:427985. - PubMed
-
- Chen Z.J., Sun L.J. Nonproteolytic functions of ubiquitin in cell signaling. Mol Cell. 2009;33:275–286. - PubMed
-
- Mukhopadhyay D., Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315:201–205. - PubMed
-
- Tompa P., Davey N.E., Gibson T.J., Babu M.M. A million peptide motifs for the molecular biologist. Mol Cell. 2014;55:161–169. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

