Sleep Drive Is Encoded by Neural Plastic Changes in a Dedicated Circuit
- PMID: 27212237
- PMCID: PMC4892967
- DOI: 10.1016/j.cell.2016.04.013
Sleep Drive Is Encoded by Neural Plastic Changes in a Dedicated Circuit
Abstract
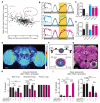
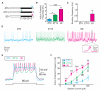
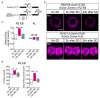
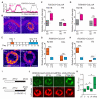
Prolonged wakefulness leads to an increased pressure for sleep, but how this homeostatic drive is generated and subsequently persists is unclear. Here, from a neural circuit screen in Drosophila, we identify a subset of ellipsoid body (EB) neurons whose activation generates sleep drive. Patch-clamp analysis indicates these EB neurons are highly sensitive to sleep loss, switching from spiking to burst-firing modes. Functional imaging and translational profiling experiments reveal that elevated sleep need triggers reversible increases in cytosolic Ca(2+) levels, NMDA receptor expression, and structural markers of synaptic strength, suggesting these EB neurons undergo "sleep-need"-dependent plasticity. Strikingly, the synaptic plasticity of these EB neurons is both necessary and sufficient for generating sleep drive, indicating that sleep pressure is encoded by plastic changes within this circuit. These studies define an integrator circuit for sleep homeostasis and provide a mechanism explaining the generation and persistence of sleep drive.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Do Flies Count Sheep or NMDA Receptors to Go to Sleep?Cell. 2016 Jun 2;165(6):1310-1311. doi: 10.1016/j.cell.2016.05.059. Cell. 2016. PMID: 27259141
References
-
- Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog. Neurobiol. 1995;45:347–360. - PubMed
-
- Berridge KC. Motivation concepts in behavioral neuroscience. Physiol. & Behav. 2004;81:179–209. - PubMed
-
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. - PubMed
-
- Borbely AA. A two process model of sleep regulation. Hum. Neurobiol. 1982;1:195–204. - PubMed
-
- Borst A, Helmstaedter M. Common circuit design in fly and mammalian motion vision. Nat. Neurosci. 2015;18:1067–1076. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

