Mustard vesicant-induced lung injury: Advances in therapy
- PMID: 27212445
- PMCID: PMC5119915
- DOI: 10.1016/j.taap.2016.05.014
Mustard vesicant-induced lung injury: Advances in therapy
Abstract
Most mortality and morbidity following exposure to vesicants such as sulfur mustard is due to pulmonary toxicity. Acute injury is characterized by epithelial detachment and necrosis in the pharynx, trachea and bronchioles, while long-term consequences include fibrosis and, in some instances, cancer. Current therapies to treat mustard poisoning are primarily palliative and do not target underlying pathophysiologic mechanisms. New knowledge about vesicant-induced pulmonary disease pathogenesis has led to the identification of potentially efficacious strategies to reduce injury by targeting inflammatory cells and mediators including reactive oxygen and nitrogen species, proteases and proinflammatory/cytotoxic cytokines. Therapeutics under investigation include corticosteroids, N-acetyl cysteine, which has both mucolytic and antioxidant properties, inducible nitric oxide synthase inhibitors, liposomes containing superoxide dismutase, catalase, and/or tocopherols, protease inhibitors, and cytokine antagonists such as anti-tumor necrosis factor (TNF)-α antibody and pentoxifylline. Antifibrotic and fibrinolytic treatments may also prove beneficial in ameliorating airway obstruction and lung remodeling. More speculative approaches include inhibitors of transient receptor potential channels, which regulate pulmonary epithelial cell membrane permeability, non-coding RNAs and mesenchymal stem cells. As mustards represent high priority chemical threat agents, identification of effective therapeutics for mitigating toxicity is highly significant.
Keywords: Fibrosis; Inflammatory mediators; Lung injury; Mustard gas; Therapeutic approaches; Vesicant.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
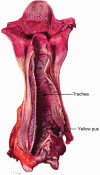
“The characteristic feature is the sloughing of the tracheal mucous membrane. The reddening of the base of the tongue and of the pharynx, with a sharp delimitation where the oesophagus has refused ingress to the toxic vapor, is seen also with chlorine and other irritant gasses. But the pharyngeal inflammation with mustard gas may proceed further to a local ulceration that will cause dysphagia for many days.”
“The mucous membrane of the trachea and bronchi is affected by the di-chlor-ethyl-sulphide in much the same way as the skin. It reacts with an intense inflammation, and death of the surface layers soon results. The mass of necrotic tissue, exuded fibrin, and pus cells may forms a yellowish-grey slough in which all manner of organisms flourish. Subsequently this false membrane comes away in patches or in entire casts from the raw surface of the bronchial wall.”
“Meantime the infected debris and secretions tend to accumulate in the bronchial ramifications at the bases of the lungs, and infection may spread from them into the lung tissues and alveoli. Septic broncho-pneumonia, localised abscesses, superficial pleurisy, and even empyema or pyropneumonthorax then develop and cause death.”
“The drawing is of a trachea at the twelfth day after gassing. The base of the tongue and the pharynx show characteristic inflammation. Yellow necrotic sloughs lie on the larynx and at the bifurcation of the trachea. Between these the trachea is red and glistening, because it is now completely denuded of both mucous membrane and of slough. The dotted line points to a little group of ulcers on the posterior wall from which bleeding has occurred. The trachea and bronchi contained an abundance of this yellow pus.”
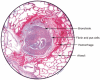
“The bronchiole is filled with fibrin and pus cells, and its lining epithelium has been completely destroyed. The inflammation has caused a characteristic ring of haemorrhage in the tissues around the bronchial tube, and infection is beginning to appear in the alveoli nearest to these inflamed tissues. But there is no generalized pulmonary oedema and no disruptive emphysema.”
“Di-chlor-ethyl-sulphide [SM] may cause some catarrhal desquamation of the pulmonary endothelial cells, but it rarely excites an outpouring of oedema fluid from the pulmonary vessels. The pathological changes in the bronchioles and in the alveoli are therefore in the sharpest contrast with those caused by phosgene. As infection spreads into the lung tissues, patches of septic bronchopneumonia and small absesses develop, and these often excite an inflammatory oedema around them.”
“If the patient lives, his bronchial mucous membrane is slowly regenerated; and during this time he is naturally subject to reflex spasms of coughing or even protracted bronchitis.”
References
-
- Aasted A, Darre E, Wulf HC. Mustard gas: clinical, toxicological, and mutagenic aspects based on modern experience. Ann. Plast. Surg. 1987;19:330–333. - PubMed
-
- Abbott-Banner K, Poll C, Verkuyl JM. Targeting TRP channels in airway disorders. Curr. Top. Med. Chem. 2013;13:310–321. - PubMed
-
- Adelipour M, Imani Fooladi AA, Yazdani S, Vahedi E, Ghanei M, Nourani MR. Smad molecules expression pattern in human bronchial airway induced by sulfur mustard. Iran. J. Allergy Asthma Immunol. 2011;10:147–154. - PubMed
-
- Aghanouri R, Ghanei M, Aslani J, Keivani-Amine H, Rastegar F, Karkhane A. Fibrogenic cytokine levels in bronchoalveolar lavage aspirates 15 years after exposure to sulfur mustard. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;287:L1160–1164. - PubMed
-
- Akyurekli C, Le Y, Richardson RB, Fergusson D, Tay J, Allan DS. A systematic review of preclinical studies on the therapeutic potential of mesenchymal stromal cell-derived microvesicles. Stem Cell. Rev. 2015;11:150–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

