Th1 versus Th2 T cell polarization by whole-cell and acellular childhood pertussis vaccines persists upon re-immunization in adolescence and adulthood
- PMID: 27212461
- PMCID: PMC4899275
- DOI: 10.1016/j.cellimm.2016.05.002
Th1 versus Th2 T cell polarization by whole-cell and acellular childhood pertussis vaccines persists upon re-immunization in adolescence and adulthood
Abstract
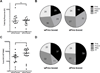
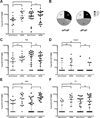
The recent increase in cases of whooping cough among teenagers in the US suggests that the acellular Bordetella pertussis vaccine (aP) that became standard in the mid 1990s might be relatively less effective than the whole-bacteria formulation (wP) previously used since the 1950s. To understand this effect, we compared antibody and T cell responses to a booster immunization in subjects who received either the wP or aP vaccine as their initial priming dose in childhood. Antibody responses in wP- and aP-primed donors were similar. Magnitude of T cell responses was higher in aP-primed individuals. Epitope mapping revealed the T cell immunodominance patterns were similar for both vaccines. Further comparison of the ratios of IFNγ and IL-5 revealed that IFNγ strongly dominates the T cell response in wP-primed donors, while IL-5 is dominant in aP primed individuals. Surprisingly, this differential pattern is maintained after booster vaccination, at times from eighteen years to several decades after the original aP/wP priming. These findings suggest that childhood aP versus wP vaccination induces functionally different T cell responses to pertussis that become fixed and are unchanged even upon boosting.
Keywords: Epitope; Pertussis; Re-immunization; T cell polarization; Vaccine.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no conflict of interest.
Figures



References
-
- Pertussis Cases by Year. Vol. 6. Center for Disease Control, Marc; 2015.
-
- Pertussis Outbreak Trends. Center for Disease Control; 2015.
-
- Cody CL, Baraff LJ, Cherry JD, Marcy SM, Manclark CR. Nature and rates of adverse reactions associated with DTP and DT immunizations in infants and children. Pediatrics. 1981;68:650–660. - PubMed
-
- Sato Y, Sato H. Development of acellular pertussis vaccines. Biologicals : journal of the International Association of Biological Standardization. 1999;27:61–69. - PubMed
-
- Report C. FDA approved of use of diphtheria and tetanus toxoid and acellular pertussis vaccine. JAMA. 1992:485. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

