Effect of High, Medium, and Low Molecular Weight Hyaluronan on Inflammation and Oxidative Stress in an In Vitro Model of Human Nasal Epithelial Cells
- PMID: 27212811
- PMCID: PMC4860232
- DOI: 10.1155/2016/8727289
Effect of High, Medium, and Low Molecular Weight Hyaluronan on Inflammation and Oxidative Stress in an In Vitro Model of Human Nasal Epithelial Cells
Erratum in
-
Corrigendum to "Effect of High, Medium, and Low Molecular Weight Hyaluronan on Inflammation and Oxidative Stress in an In Vitro Model of Human Nasal Epithelial Cells".Mediators Inflamm. 2019 Oct 3;2019:9198518. doi: 10.1155/2019/9198518. eCollection 2019. Mediators Inflamm. 2019. PMID: 31686987 Free PMC article.
Abstract
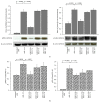
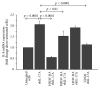
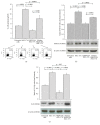
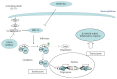
IL-17A is involved in the activation of oxidative stress and inflammation in nasal epithelial cells. Hyaluronan (HA) in its high molecular weight form (HMW-HA) shows anti-inflammatory responses in contrast to low and medium molecular weight HA (LMW-HA and MMW-HA). The aim of this study was to investigate the pro- or anti-inflammatory biologic function of HA at different molecular weight in an in vitro model of nasal inflammation IL-17A mediated. We evaluated the ERK1/2 and IκBα phosphorylation, NF-κB signal pathway activation, ROS production, IL-8 and NOX-4 protein, and mRNA levels, in nasal epithelial cells RPMI 2650 stimulated with recombinant human (rh) IL-17A. Furthermore, the cells were treated with HMW-HA, MMW-HA, LMW-HA, and U0126. Our results showed that rhIL-17A increased the ERK1/2, IκBα phosphorylation and NF-κB signal pathway activation, ROS production, IL-8 and NOX-4 proteins, and mRNA levels. The addiction of HMW-HA or U0126 showed a significant downregulatory effect on inflammation due to the rhIL-17A stimulation in nasal epithelial cells. IL-17A is able to generate oxidative stress and inflammation via the activation of ERK1/2/NF-κB pathway in nasal epithelial cells. The HMW-HA might represent a coadjuvant of the classic anti-inflammatory/antioxidative treatment of nasal epithelial cells during IL-17A nasal inflammation.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

