Denaturation and in Vitro Gastric Digestion of Heat-Treated Quinoa Protein Isolates Obtained at Various Extraction pH
- PMID: 27212897
- PMCID: PMC4851711
- DOI: 10.1007/s11483-016-9429-4
Denaturation and in Vitro Gastric Digestion of Heat-Treated Quinoa Protein Isolates Obtained at Various Extraction pH
Abstract
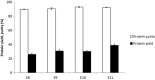
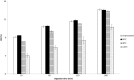
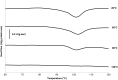
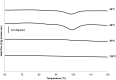
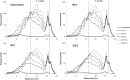
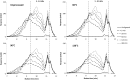
The aim of this study was to determine the influence of heat processing on denaturation and digestibility properties of protein isolates obtained from sweet quinoa (Chenopodium quinoa Willd) at various extraction pH values (8, 9, 10 and 11). Pretreatment of suspensions of protein isolates at 60, 90 and 120 °C for 30 min led to protein denaturation and aggregation, which was enhanced at higher treatment temperatures. The in vitro gastric digestibility measured during 6 h was lower for protein extracts pre-treated at 90 and 120 °C compared to 60 °C. The digestibility decreased with increasing extraction pH, which could be ascribed to protein aggregation. Protein digestibility of the quinoa protein isolates was higher compared to wholemeal quinoa flour. We conclude that an interactive effect of processing temperature and extraction pH on in vitro gastric digestibility of quinoa protein isolates obtained at various extraction pH is observed. This gives a first indication of how the nutritional value of quinoa protein could be influenced by heat processing, protein extraction conditions and other grain components.
Keywords: Denaturation; Digestibility; Extraction pH; Heat processing; Protein; Quinoa.
Figures










References
-
- Limburg H, Masterbroek HD Breeding high yielding lines of Chenopodium quinoa Willd. with saponin free seed. In: Stølen O, Bruhn K, Pithan K, Hill J (eds) Proc COST 814 workshop on Small Grain Cereals and Pseudo-Cereals, Copenhagen, 24 February 1997. pp 103–114
-
- Mastebroek HD, van Loo EN, Dolstra O. Combining ability for seed yield traits of Chenopodium quinoa breeding lines. Euphytica. 2002;125(3):427–432. doi: 10.1023/A:1016030129541. - DOI
-
- Avila Ruiz G, Xiao W, van Boekel M, Stieger M, Minor M (2016) Effect of extraction pH on heat-induced aggregation, gelation and microstructure of protein from sweet quinoa (Chenopodium quinoa Willd) (submitted) - PubMed
-
- Valenzuela C, Abugoch L, Tapia C, Gamboa A. Effect of alkaline extraction on the structure of the protein of quinoa (Chenopodium quinoa willd.) and its influence on film formation. Int J Food Sci Tech. 2013;48(4):843–849. doi: 10.1111/ijfs.12035. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
