Cell proliferation and apoptosis in optic nerve and brain integration centers of adult trout Oncorhynchus mykiss after optic nerve injury
- PMID: 27212918
- PMCID: PMC4870914
- DOI: 10.4103/1673-5374.180742
Cell proliferation and apoptosis in optic nerve and brain integration centers of adult trout Oncorhynchus mykiss after optic nerve injury
Abstract
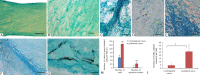
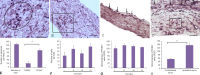
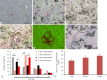
Fishes have remarkable ability to effectively rebuild the structure of nerve cells and nerve fibers after central nervous system injury. However, the underlying mechanism is poorly understood. In order to address this issue, we investigated the proliferation and apoptosis of cells in contralateral and ipsilateral optic nerves, after stab wound injury to the eye of an adult trout Oncorhynchus mykiss. Heterogenous population of proliferating cells was investigated at 1 week after injury. TUNEL labeling gave a qualitative and quantitative assessment of apoptosis in the cells of optic nerve of trout 2 days after injury. After optic nerve injury, apoptotic response was investigated, and mass patterns of cell migration were found. The maximal concentration of apoptotic bodies was detected in the areas of mass clumps of cells. It is probably indicative of massive cell death in the area of high phagocytic activity of macrophages/microglia. At 1 week after optic nerve injury, we observed nerve cell proliferation in the trout brain integration centers: the cerebellum and the optic tectum. In the optic tectum, proliferating cell nuclear antigen (PCNA)-immunopositive radial glia-like cells were identified. Proliferative activity of nerve cells was detected in the dorsal proliferative (matrix) area of the cerebellum and in parenchymal cells of the molecular and granular layers whereas local clusters of undifferentiated cells which formed neurogenic niches were observed in both the optic tectum and cerebellum after optic nerve injury. In vitro analysis of brain cells of trout showed that suspension cells compared with monolayer cells retain higher proliferative activity, as evidenced by PCNA immunolabeling. Phase contrast observation showed mitosis in individual cells and the formation of neurospheres which gradually increased during 1-4 days of culture. The present findings suggest that trout can be used as a novel model for studying neuronal regeneration.
Keywords: apoptosis; brain; nerve regeneration; neural regeneration; neurogenic niches; neurospheres; optic nerve; proliferation; radial glia cells.
Conflict of interest statement
Figures



Similar articles
-
Neurolin expression in the optic nerve and immunoreactivity of Pax6-positive niches in the brain of rainbow trout (Oncorhynchus mykiss) after unilateral eye injury.Neural Regen Res. 2019 Jan;14(1):156-171. doi: 10.4103/1673-5374.243721. Neural Regen Res. 2019. PMID: 30531090 Free PMC article.
-
GFAP expression in the optic nerve and increased H2S generation in the integration centers of the rainbow trout (Oncorhynchus mykiss) brain after unilateral eye injury.Neural Regen Res. 2020 Oct;15(10):1867-1886. doi: 10.4103/1673-5374.280320. Neural Regen Res. 2020. PMID: 32246635 Free PMC article.
-
[Reparative Neurogenesis in the Brain and Changes in the Optic Nerve of Adult Trout Oncorhynchus mykiss after Mechanical Damage of the Eye].Ontogenez. 2016 Jan-Feb;47(1):15-39. Ontogenez. 2016. PMID: 27149746 Russian.
-
Astrocytes as gate-keepers in optic nerve regeneration--a mini-review.Comp Biochem Physiol A Mol Integr Physiol. 2009 Feb;152(2):135-8. doi: 10.1016/j.cbpa.2008.09.026. Epub 2008 Oct 2. Comp Biochem Physiol A Mol Integr Physiol. 2009. PMID: 18930160 Review.
-
A molecular mechanism of optic nerve regeneration in fish: the retinoid signaling pathway.Prog Retin Eye Res. 2013 Nov;37:13-30. doi: 10.1016/j.preteyeres.2013.07.004. Epub 2013 Aug 28. Prog Retin Eye Res. 2013. PMID: 23994437 Review.
Cited by
-
Regenerating reptile retinas: a comparative approach to restoring retinal ganglion cell function.Eye (Lond). 2017 Feb;31(2):167-172. doi: 10.1038/eye.2016.224. Epub 2016 Nov 11. Eye (Lond). 2017. PMID: 27834958 Free PMC article. Review.
-
Neurolin expression in the optic nerve and immunoreactivity of Pax6-positive niches in the brain of rainbow trout (Oncorhynchus mykiss) after unilateral eye injury.Neural Regen Res. 2019 Jan;14(1):156-171. doi: 10.4103/1673-5374.243721. Neural Regen Res. 2019. PMID: 30531090 Free PMC article.
-
Constitutive Neurogenesis and Neuronal Plasticity in the Adult Cerebellum and Brainstem of Rainbow Trout, Oncorhynchus mykiss.Int J Mol Sci. 2024 May 21;25(11):5595. doi: 10.3390/ijms25115595. Int J Mol Sci. 2024. PMID: 38891784 Free PMC article.
-
GFAP expression in the optic nerve and increased H2S generation in the integration centers of the rainbow trout (Oncorhynchus mykiss) brain after unilateral eye injury.Neural Regen Res. 2020 Oct;15(10):1867-1886. doi: 10.4103/1673-5374.280320. Neural Regen Res. 2020. PMID: 32246635 Free PMC article.
References
-
- Adolf B, Chapouton P, Lam CS, Topp S, Tannhäuser B, Strähle U, Götz M, Bally-Cuif L. Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon. Devel Biol. 2006;295:278–293. - PubMed
-
- Andral R, Hurard C, Elziere-Papayanni P, Vivares CP. Establishment and characterization of a rainbow trout kidney cell-line, RTK Montpellier. In: Perkins FO, Cheng CC, editors. Pathology in Marine Science. San Diego, USA: Academic Press; 1990. pp. 33–42.
-
- Arévalo R, Alonso JR, García-Ojeda E, Briñón JG, Crespo C, Aijón J. NADPH-diaphorase in the central nervous system of the tench (Tinca tinca L., 1758) J Comp Neurol. 1995;352:398–420. - PubMed
-
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

