Flavonoids Extraction from Propolis Attenuates Pathological Cardiac Hypertrophy through PI3K/AKT Signaling Pathway
- PMID: 27213000
- PMCID: PMC4860246
- DOI: 10.1155/2016/6281376
Flavonoids Extraction from Propolis Attenuates Pathological Cardiac Hypertrophy through PI3K/AKT Signaling Pathway
Abstract
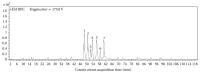
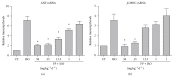
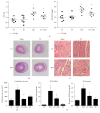
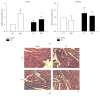
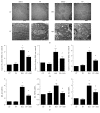
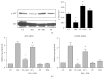
Propolis, a traditional medicine, has been widely used for a thousand years as an anti-inflammatory and antioxidant drug. The flavonoid fraction is the main active component of propolis, which possesses a wide range of biological activities, including activities related to heart disease. However, the role of the flavonoids extraction from propolis (FP) in heart disease remains unknown. This study shows that FP could attenuate ISO-induced pathological cardiac hypertrophy (PCH) and heart failure in mice. The effect of the two fetal cardiac genes, atrial natriuretic factor (ANF) and β-myosin heavy chain (β-MHC), on PCH was reversed by FP. Echocardiography analysis revealed cardiac ventricular dilation and contractile dysfunction in ISO-treated mice. This finding is consistent with the increased heart weight and cardiac ANF protein levels, massive replacement fibrosis, and myocardial apoptosis. However, pretreatment of mice with FP could attenuate cardiac dysfunction and hypertrophy in vivo. Furthermore, the cardiac protection of FP was suppressed by the pan-PI3K inhibitor wortmannin. FP is a novel cardioprotective agent that can attenuate adverse cardiac dysfunction, hypertrophy, and associated disorder, such as fibrosis. The effects may be closely correlated with PI3K/AKT signaling. FP may be clinically used to inhibit PCH progression and heart failure.
Figures
References
-
- Allwood M. A., Kinobe R. T., Ballantyne L., et al. Heme oxygenase-1 overexpression exacerbates heart failure with aging and pressure overload but is protective against isoproterenol-induced cardiomyopathy in mice. Cardiovascular Pathology. 2014;23(4):231–237. doi: 10.1016/j.carpath.2014.03.007. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

