Nanoscale Semiconductor Devices as New Biomaterials
- PMID: 27213041
- PMCID: PMC4874554
- DOI: 10.1039/C3BM60280J
Nanoscale Semiconductor Devices as New Biomaterials
Abstract
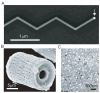
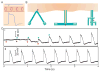
Research on nanoscale semiconductor devices will elicit a novel understanding of biological systems. First, we discuss why it is necessary to build interfaces between cells and semiconductor nanoelectronics. Second, we describe some recent molecular biophysics studies with nanowire field effect transistor sensors. Third, we present the use of nanowire transistors as electrical recording devices that can be integrated into synthetic tissues and targeted intra- or extracellularly to study single cells. Lastly, we discuss future directions and challenges in further developing this area of research, which will advance biology and medicine.
Figures


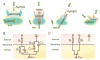

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

