Molecular Mechanisms of Pulmonary Vascular Remodeling in Pulmonary Arterial Hypertension
- PMID: 27213345
- PMCID: PMC4881582
- DOI: 10.3390/ijms17050761
Molecular Mechanisms of Pulmonary Vascular Remodeling in Pulmonary Arterial Hypertension
Abstract
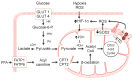
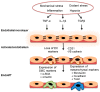
Pulmonary arterial hypertension (PAH) is a devastating disease that is precipitated by hypertrophic pulmonary vascular remodeling of distal arterioles to increase pulmonary artery pressure and pulmonary vascular resistance in the absence of left heart, lung parenchymal, or thromboembolic disease. Despite available medical therapy, pulmonary artery remodeling and its attendant hemodynamic consequences result in right ventricular dysfunction, failure, and early death. To limit morbidity and mortality, attention has focused on identifying the cellular and molecular mechanisms underlying aberrant pulmonary artery remodeling to identify pathways for intervention. While there is a well-recognized heritable genetic component to PAH, there is also evidence of other genetic perturbations, including pulmonary vascular cell DNA damage, activation of the DNA damage response, and variations in microRNA expression. These findings likely contribute, in part, to dysregulation of proliferation and apoptosis signaling pathways akin to what is observed in cancer; changes in cellular metabolism, metabolic flux, and mitochondrial function; and endothelial-to-mesenchymal transition as key signaling pathways that promote pulmonary vascular remodeling. This review will highlight recent advances in the field with an emphasis on the aforementioned molecular mechanisms as contributors to the pulmonary vascular disease pathophenotype.
Keywords: DNA damage; endothelial-to-mesenchymal transition; metabolism; microRNA; mitochondria; pulmonary arterial hypertension.
Figures
References
-
- Ling Y., Johnson M.K., Kiely D.G., Condliffe R., Elliot C.A., Gibbs J.S., Howard L.S., Pepke-Zaba J., Sheares K.K., Corris P.A., et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: Results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am. J. Respir. Crit. Care Med. 2012;186:790–796. doi: 10.1164/rccm.201203-0383OC. - DOI - PubMed
-
- Tuder R.M., Archer S.L., Dorfmuller P., Erzurum S.C., Guignabert C., Michelakis E., Rabinovitch M., Schermuly R., Stenmark K.R., Morrell N.W. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J. Am. Coll. Cardiol. 2013;62:D4–D12. doi: 10.1016/j.jacc.2013.10.025. - DOI - PMC - PubMed
-
- International P.P.H.C., Lane K.B., Machado R.D., Pauciulo M.W., Thomson J.R., Phillips J.A., 3rd, Loyd J.E., Nichols W.C., Trembath R.C. Heterozygous germline mutations in BMPR2, encoding a TGF-β receptor, cause familial primary pulmonary hypertension. Nat. Genet. 2000;26:81–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

