Functional and Biochemical Characterization of Three Recombinant Human Glucose-6-Phosphate Dehydrogenase Mutants: Zacatecas, Vanua-Lava and Viangchan
- PMID: 27213370
- PMCID: PMC4881603
- DOI: 10.3390/ijms17050787
Functional and Biochemical Characterization of Three Recombinant Human Glucose-6-Phosphate Dehydrogenase Mutants: Zacatecas, Vanua-Lava and Viangchan
Abstract
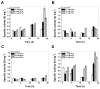
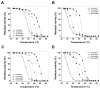
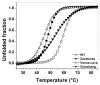
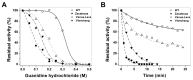
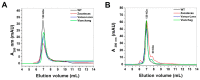
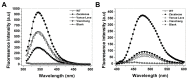
Glucose-6-phosphate dehydrogenase (G6PD) deficiency in humans causes severe disease, varying from mostly asymptomatic individuals to patients showing neonatal jaundice, acute hemolysis episodes or chronic nonspherocytic hemolytic anemia. In order to understand the effect of the mutations in G6PD gene function and its relation with G6PD deficiency severity, we report the construction, cloning and expression as well as the detailed kinetic and stability characterization of three purified clinical variants of G6PD that present in the Mexican population: G6PD Zacatecas (Class I), Vanua-Lava (Class II) and Viangchan (Class II). For all the G6PD mutants, we obtained low purification yield and altered kinetic parameters compared with Wild Type (WT). Our results show that the mutations, regardless of the distance from the active site where they are located, affect the catalytic properties and structural parameters and that these changes could be associated with the clinical presentation of the deficiency. Specifically, the structural characterization of the G6PD Zacatecas mutant suggests that the R257L mutation have a strong effect on the global stability of G6PD favoring an unstable active site. Using computational analysis, we offer a molecular explanation of the effects of these mutations on the active site.
Keywords: glucose-6-phosphate dehydrogenase (G6PD) deficiency; human G6PD mutants; steady state kinetics; structural characterization; thermostability.
Figures


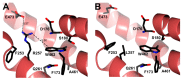
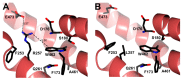
Similar articles
-
Effects of Single and Double Mutants in Human Glucose-6-Phosphate Dehydrogenase Variants Present in the Mexican Population: Biochemical and Structural Analysis.Int J Mol Sci. 2020 Apr 15;21(8):2732. doi: 10.3390/ijms21082732. Int J Mol Sci. 2020. PMID: 32326520 Free PMC article.
-
Long-range structural defects by pathogenic mutations in most severe glucose-6-phosphate dehydrogenase deficiency.Proc Natl Acad Sci U S A. 2021 Jan 26;118(4):e2022790118. doi: 10.1073/pnas.2022790118. Proc Natl Acad Sci U S A. 2021. PMID: 33468660 Free PMC article.
-
A trade off between catalytic activity and protein stability determines the clinical manifestations of glucose-6-phosphate dehydrogenase (G6PD) deficiency.Int J Biol Macromol. 2017 Nov;104(Pt A):145-156. doi: 10.1016/j.ijbiomac.2017.06.002. Epub 2017 Jun 3. Int J Biol Macromol. 2017. PMID: 28583873 Free PMC article.
-
An Overall View of the Functional and Structural Characterization of Glucose-6-Phosphate Dehydrogenase Variants in the Mexican Population.Int J Mol Sci. 2023 Aug 11;24(16):12691. doi: 10.3390/ijms241612691. Int J Mol Sci. 2023. PMID: 37628871 Free PMC article. Review.
-
Glucose-6-Phosphate Dehydrogenase Deficiency: An Overview of the Prevalence and Genetic Variants in Saudi Arabia.Hemoglobin. 2021 Sep;45(5):287-295. doi: 10.1080/03630269.2022.2034644. Epub 2022 Feb 13. Hemoglobin. 2021. PMID: 35156495 Review.
Cited by
-
Preliminary Study of Structural Changes of Glucose-6-Phosphate Dehydrogenase Deficiency Variants.Biomedicine (Taipei). 2022 Sep 1;12(3):12-19. doi: 10.37796/2211-8039.1355. eCollection 2022. Biomedicine (Taipei). 2022. PMID: 36381187 Free PMC article.
-
Coupling between Protein Stability and Catalytic Activity Determines Pathogenicity of G6PD Variants.Cell Rep. 2017 Mar 14;18(11):2592-2599. doi: 10.1016/j.celrep.2017.02.048. Cell Rep. 2017. PMID: 28297664 Free PMC article.
-
Evaluation of Three Mutations in Codon 385 of Glucose-6-Phosphate Dehydrogenase via Biochemical and In Silico Analysis.Int J Mol Sci. 2024 Nov 22;25(23):12556. doi: 10.3390/ijms252312556. Int J Mol Sci. 2024. PMID: 39684266 Free PMC article.
-
Gene Cloning, Recombinant Expression, Characterization, and Molecular Modeling of the Glycolytic Enzyme Triosephosphate Isomerase from Fusarium oxysporum.Microorganisms. 2019 Dec 24;8(1):40. doi: 10.3390/microorganisms8010040. Microorganisms. 2019. PMID: 31878282 Free PMC article.
-
Glucose-6-Phosphate Dehydrogenase Deficiency and Neonatal Hyperbilirubinemia: Insights on Pathophysiology, Diagnosis, and Gene Variants in Disease Heterogeneity.Front Pediatr. 2022 May 24;10:875877. doi: 10.3389/fped.2022.875877. eCollection 2022. Front Pediatr. 2022. PMID: 35685917 Free PMC article. Review.
References
-
- Pai G.S., Sprenkle J.A., Do T.T., Mareni C.E., Migeon B.R. Localization of loci for hypoxanthine phosphoribosyltransferase and glucose-6-phosphate dehydrogenase and biochemical evidence of nonrandom X chromosome expression from studies of a human X-autosome translocation. Proc. Natl. Acad. Sci. USA. 1980;77:2810–2813. doi: 10.1073/pnas.77.5.2810. - DOI - PMC - PubMed
-
- Beutler E. G6PD deficiency. Blood. 1994;84:3613–3636. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

