Recognizing Physisorption and Chemisorption in Carbon Nanotubes Gas Sensors by Double Exponential Fitting of the Response
- PMID: 27213387
- PMCID: PMC4883422
- DOI: 10.3390/s16050731
Recognizing Physisorption and Chemisorption in Carbon Nanotubes Gas Sensors by Double Exponential Fitting of the Response
Abstract
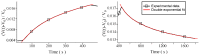
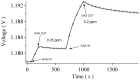
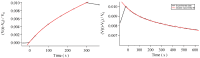
Multi-walled carbon nanotubes (CNTs) have been grown in situ on a SiO 2 substrate and used as gas sensors. For this purpose, the voltage response of the CNTs as a function of time has been used to detect H 2 and CO 2 at various concentrations by supplying a constant current to the system. The analysis of both adsorptions and desorptions curves has revealed two different exponential behaviours for each curve. The study of the characteristic times, obtained from the fitting of the data, has allowed us to identify separately chemisorption and physisorption processes on the CNTs.
Keywords: CNTs sensors; chemisorption; physisorption.
Figures




References
-
- Wang Y., Yeow J.T. A review of carbon nanotubes-based gas sensors. J. Sens. 2009;2009:1–24. doi: 10.1155/2009/493904. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

