Apigenin Ameliorates Dyslipidemia, Hepatic Steatosis and Insulin Resistance by Modulating Metabolic and Transcriptional Profiles in the Liver of High-Fat Diet-Induced Obese Mice
- PMID: 27213439
- PMCID: PMC4882717
- DOI: 10.3390/nu8050305
Apigenin Ameliorates Dyslipidemia, Hepatic Steatosis and Insulin Resistance by Modulating Metabolic and Transcriptional Profiles in the Liver of High-Fat Diet-Induced Obese Mice
Abstract
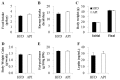
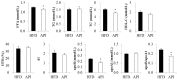
Several in vitro and in vivo studies have reported the anti-inflammatory, anti-diabetic and anti-obesity effects of the flavonoid apigenin. However, the long-term supplementary effects of low-dose apigenin on obesity are unclear. Therefore, we investigated the protective effects of apigenin against obesity and related metabolic disturbances by exploring the metabolic and transcriptional responses in high-fat diet (HFD)-induced obese mice. C57BL/6J mice were fed an HFD or apigenin (0.005%, w/w)-supplemented HFD for 16 weeks. In HFD-fed mice, apigenin lowered plasma levels of free fatty acid, total cholesterol, apolipoprotein B and hepatic dysfunction markers and ameliorated hepatic steatosis and hepatomegaly, without altering food intake and adiposity. These effects were partly attributed to upregulated expression of genes regulating fatty acid oxidation, tricarboxylic acid cycle, oxidative phosphorylation, electron transport chain and cholesterol homeostasis, downregulated expression of lipolytic and lipogenic genes and decreased activities of enzymes responsible for triglyceride and cholesterol ester synthesis in the liver. Moreover, apigenin lowered plasma levels of pro-inflammatory mediators and fasting blood glucose. The anti-hyperglycemic effect of apigenin appeared to be related to decreased insulin resistance, hyperinsulinemia and hepatic gluconeogenic enzymes activities. Thus, apigenin can ameliorate HFD-induced comorbidities via metabolic and transcriptional modulations in the liver.
Keywords: apigenin; hepatic metabolic and transcriptional responses; hepatic steatosis; high-fat diet-induced obesity; insulin resistance.
Figures




Similar articles
-
Long-term dietary supplementation with low-dose nobiletin ameliorates hepatic steatosis, insulin resistance, and inflammation without altering fat mass in diet-induced obesity.Mol Nutr Food Res. 2017 Aug;61(8). doi: 10.1002/mnfr.201600889. Epub 2017 Mar 2. Mol Nutr Food Res. 2017. PMID: 28116779
-
Effect of Green Tea Extract on Systemic Metabolic Homeostasis in Diet-Induced Obese Mice Determined via RNA-Seq Transcriptome Profiles.Nutrients. 2016 Oct 14;8(10):640. doi: 10.3390/nu8100640. Nutrients. 2016. PMID: 27754422 Free PMC article.
-
Seabuckthorn Leaves Extract and Flavonoid Glycosides Extract from Seabuckthorn Leaves Ameliorates Adiposity, Hepatic Steatosis, Insulin Resistance, and Inflammation in Diet-Induced Obesity.Nutrients. 2017 Jun 2;9(6):569. doi: 10.3390/nu9060569. Nutrients. 2017. PMID: 28574484 Free PMC article.
-
Role of apigenin in targeting metabolic syndrome: A systematic review.Iran J Basic Med Sci. 2024;27(5):524-534. doi: 10.22038/IJBMS.2024.71539.15558. Iran J Basic Med Sci. 2024. PMID: 38629096 Free PMC article. Review.
-
Current Status and Future Perspectives on Therapeutic Potential of Apigenin: Focus on Metabolic-Syndrome-Dependent Organ Dysfunction.Antioxidants (Basel). 2021 Oct 19;10(10):1643. doi: 10.3390/antiox10101643. Antioxidants (Basel). 2021. PMID: 34679777 Free PMC article. Review.
Cited by
-
Improvement of Obesity and Dyslipidemic Activity of Amomum tsao-ko in C57BL/6 Mice Fed a High-Carbohydrate Diet.Molecules. 2021 Mar 15;26(6):1638. doi: 10.3390/molecules26061638. Molecules. 2021. PMID: 33804179 Free PMC article.
-
Pharmacological and Molecular Insight on the Cardioprotective Role of Apigenin.Nutrients. 2023 Jan 12;15(2):385. doi: 10.3390/nu15020385. Nutrients. 2023. PMID: 36678254 Free PMC article. Review.
-
Clinical applications and mechanism insights of natural flavonoids against type 2 diabetes mellitus.Heliyon. 2024 Apr 16;10(9):e29718. doi: 10.1016/j.heliyon.2024.e29718. eCollection 2024 May 15. Heliyon. 2024. PMID: 38694079 Free PMC article. Review.
-
Chamomile as a potential remedy for obesity and metabolic syndrome.EXCLI J. 2021 Jul 26;20:1261-1286. doi: 10.17179/excli2021-4013. eCollection 2021. EXCLI J. 2021. PMID: 34602925 Free PMC article. Review.
-
Sarcopenic Obesity: Involvement of Oxidative Stress and Beneficial Role of Antioxidant Flavonoids.Antioxidants (Basel). 2023 May 8;12(5):1063. doi: 10.3390/antiox12051063. Antioxidants (Basel). 2023. PMID: 37237929 Free PMC article. Review.
References
-
- Schmidt M.I., Duncan B.B., Sharrett A.R., Lindberg G., Savage P.J., Offenbacher S., Azambuja M.I., Tracy R.P., Heiss G. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): A cohort study. Lancet. 1999;353:1649–1652. doi: 10.1016/S0140-6736(99)01046-6. - DOI - PubMed
-
- Yu R., Kim C.S., Kang J.H. Inflammatory components of adipose tissue as target for treatment of metabolic syndrome. Forum Nutr. 2009;61:95–103. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

