Phase I study of pemetrexed with sorafenib in advanced solid tumors
- PMID: 27213589
- PMCID: PMC5173162
- DOI: 10.18632/oncotarget.9434
Phase I study of pemetrexed with sorafenib in advanced solid tumors
Abstract
Purpose: To determine if combination treatment with pemetrexed and sorafenib is safe and tolerable in patients with advanced solid tumors.
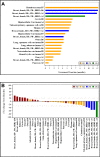
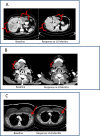
Results: Thirty-seven patients were enrolled and 36 patients were treated (24 in cohort A; 12 in cohort B). The cohort A dose schedule resulted in problematic cumulative toxicity, while the cohort B dose schedule was found to be more tolerable. The maximum tolerated dose (MTD) was pemetrexed 750 mg/m2 every 14 days with oral sorafenib 400 mg given twice daily on days 1-5. Because dosing delays and modifications were associated with the MTD, the recommended phase II dose was declared to be pemetrexed 500 mg/m2 every 14 days with oral sorafenib 400 mg given twice daily on days 1-5. Thirty-three patients were evaluated for antitumor activity. One complete response and 4 partial responses were observed (15% overall response rate). Stable disease was seen in 15 patients (45%). Four patients had a continued response at 6 months, including 2 of 5 patients with triple-negative breast cancer.
Experimental design: A phase I trial employing a standard 3 + 3 design was conducted in patients with advanced solid tumors. Cohort A involved a novel dose escalation schema exploring doses of pemetrexed every 14 days with continuous sorafenib. Cohort B involved a modified schedule of sorafenib dosing on days 1-5 of each 14-day pemetrexed cycle. Radiographic assessments were conducted every 8 weeks.
Conclusions: Pemetrexed and intermittent sorafenib therapy is a safe and tolerable combination for patients, with promising activity seen in patients with breast cancer.
Keywords: clinical trial; pemetrexed; solid tumors; sorafenib.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
References
-
- Robert NJ, Conkling PR, O'Rourke MA, Kuefler PR, McIntyre KJ, Zhan F, Asmar L, Wang Y, Shonukan OO, O'shaughnessy JA. Results of a phase II study of pemetrexed as first-line chemotherapy in patients with advanced or metastatic breast cancer. Breast Cancer Res Treat. 2011;126:101–108. - PubMed
-
- Jarmula A. Antifolate inhibitors of thymidylate synthase as anticancer drugs. Mini Rev Med Chem. 2010;10:1211–1222. - PubMed
-
- Fleeman N, Bagust A, McLeod C, Greenhalgh J, Boland A, Dundar Y, Dickson R, Tudur Smith C, Davis H, Green J, Pearson M. Pemetrexed for the first-line treatment of locally advanced or metastatic non-small cell lung cancer. Health Technol Assess. 2010;14:47–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

