Multifunctional oxidosqualene cyclases and cytochrome P450 involved in the biosynthesis of apple fruit triterpenic acids
- PMID: 27214242
- PMCID: PMC5089662
- DOI: 10.1111/nph.13996
Multifunctional oxidosqualene cyclases and cytochrome P450 involved in the biosynthesis of apple fruit triterpenic acids
Abstract
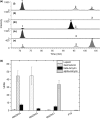
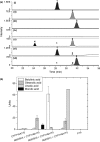
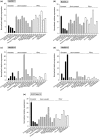
Apple (Malus × domestica) accumulates bioactive ursane-, oleanane-, and lupane-type triterpenes in its fruit cuticle, but their biosynthetic pathway is still poorly understood. We used a homology-based approach to identify and functionally characterize two new oxidosqualene cyclases (MdOSC4 and MdOSC5) and one cytochrome P450 (CYP716A175). The gene expression patterns of these enzymes and of previously described oxidosqualene cyclases were further studied in 20 apple cultivars with contrasting triterpene profiles. MdOSC4 encodes a multifunctional oxidosqualene cyclase producing an oleanane-type triterpene, putatively identified as germanicol, as well as β-amyrin and lupeol, in the proportion 82 : 14 : 4. MdOSC5 cyclizes 2,3-oxidosqualene into lupeol and β-amyrin at a ratio of 95 : 5. CYP716A175 catalyses the C-28 oxidation of α-amyrin, β-amyrin, lupeol and germanicol, producing ursolic acid, oleanolic acid, betulinic acid, and putatively morolic acid. The gene expression of MdOSC1 was linked to the concentrations of ursolic and oleanolic acid, whereas the expression of MdOSC5 was correlated with the concentrations of betulinic acid and its caffeate derivatives. Two new multifuntional triterpene synthases as well as a multifunctional triterpene C-28 oxidase were identified in Malus × domestica. This study also suggests that MdOSC1 and MdOSC5 are key genes in apple fruit triterpene biosynthesis.
Keywords: Malus × domestica; apple; betulinic acid; cytochrome P450; germanicol; oxydosqualene cyclase; triterpene; ursolic acid.
© 2016 The Authors. New Phytologist © 2016 New Phytologist Trust.
Figures




References
-
- Andre CM, Greenwood JM, Walker EG, Rassam M, Sullivan M, Evers D, Perry NB, Laing WA. 2012. Anti‐inflammatory procyanidins and triterpenes in 109 apple varieties. Journal of Agricultural and Food Chemistry 60: 10546–10554. - PubMed
-
- Andre CM, Larsen L, Burgess EJ, Jensen DJ, Cooney JM, Evers D, Zhang J, Perry NB, Laing WA. 2013. Unusual immuno‐modulatory triterpene‐caffeates in the skins of russeted varieties of apples and pears. Journal of Agricultural and Food Chemistry 61: 2773–2779. - PubMed
-
- Barton D, Brooks C. 1950. Morolic acid, a triterpenoid sapogenin. Journal of the American Chemical Society 72: 3314.
-
- Basyuni M, Oku H, Tsujimoto E, Kinjo K, Baba S, Takara K. 2007. Triterpene synthases from the Okinawan mangrove tribe, Rhizophoraceae. FEBS Journal 274: 5028–5042. - PubMed
-
- Belding RD, Blankenship SM, Young E, Leidy RB. 1998. Composition and variability of epicuticular waxes in apple cultivars. Journal of the American Society for Horticultural Science 123: 348–356.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

