Paracellular epithelial sodium transport maximizes energy efficiency in the kidney
- PMID: 27214555
- PMCID: PMC4922683
- DOI: 10.1172/JCI83942
Paracellular epithelial sodium transport maximizes energy efficiency in the kidney
Abstract
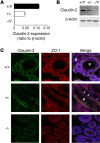
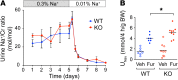
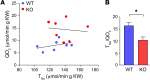
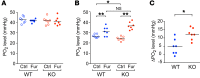
Efficient oxygen utilization in the kidney may be supported by paracellular epithelial transport, a form of passive diffusion that is driven by preexisting transepithelial electrochemical gradients. Claudins are tight-junction transmembrane proteins that act as paracellular ion channels in epithelial cells. In the proximal tubule (PT) of the kidney, claudin-2 mediates paracellular sodium reabsorption. Here, we used murine models to investigate the role of claudin-2 in maintaining energy efficiency in the kidney. We found that claudin-2-null mice conserve sodium to the same extent as WT mice, even during profound dietary sodium depletion, as a result of the upregulation of transcellular Na-K-2Cl transport activity in the thick ascending limb of Henle. We hypothesized that shifting sodium transport to transcellular pathways would lead to increased whole-kidney oxygen consumption. Indeed, compared with control animals, oxygen consumption in the kidneys of claudin-2-null mice was markedly increased, resulting in medullary hypoxia. Furthermore, tubular injury in kidneys subjected to bilateral renal ischemia-reperfusion injury was more severe in the absence of claudin-2. Our results indicate that paracellular transport in the PT is required for efficient utilization of oxygen in the service of sodium transport. We speculate that paracellular permeability may have evolved as a general strategy in epithelial tissues to maximize energy efficiency.
Figures


Comment in
-
Metabolism: Energy-efficient transport in the kidney.Nat Rev Nephrol. 2016 Aug;12(8):443. doi: 10.1038/nrneph.2016.83. Epub 2016 Jun 13. Nat Rev Nephrol. 2016. PMID: 27292105 No abstract available.
-
Paracellular transport as a strategy for energy conservation by multicellular organisms?Tissue Barriers. 2017 Apr 3;5(2):e1301852. doi: 10.1080/21688370.2017.1301852. Epub 2017 Mar 3. Tissue Barriers. 2017. PMID: 28452575 Free PMC article.
References
-
- Staehelin LA. Further observations on the fine structure of freeze-cleaved tight junctions. J Cell Sci. 1973;13(3):763–786. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

