Evaluation of ambiguous associations in the amygdala by learning the structure of the environment
- PMID: 27214568
- PMCID: PMC5655997
- DOI: 10.1038/nn.4308
Evaluation of ambiguous associations in the amygdala by learning the structure of the environment
Abstract
Recognizing predictive relationships is critical for survival, but an understanding of the underlying neural mechanisms remains elusive. In particular, it is unclear how the brain distinguishes predictive relationships from spurious ones when evidence about a relationship is ambiguous, or how it computes predictions given such uncertainty. To better understand this process, we introduced ambiguity into an associative learning task by presenting aversive outcomes both in the presence and in the absence of a predictive cue. Electrophysiological and optogenetic approaches revealed that amygdala neurons directly regulated and tracked the effects of ambiguity on learning. Contrary to established accounts of associative learning, however, interference from competing associations was not required to assess an ambiguous cue-outcome contingency. Instead, animals' behavior was explained by a normative account that evaluates different models of the environment's statistical structure. These findings suggest an alternative view of amygdala circuits in resolving ambiguity during aversive learning.
Conflict of interest statement
Figures
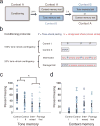
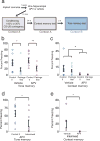
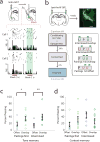
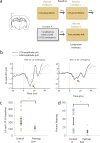

Similar articles
-
A feedback neural circuit for calibrating aversive memory strength.Nat Neurosci. 2017 Jan;20(1):90-97. doi: 10.1038/nn.4439. Epub 2016 Nov 14. Nat Neurosci. 2017. PMID: 27842071
-
Optical activation of lateral amygdala pyramidal cells instructs associative fear learning.Proc Natl Acad Sci U S A. 2010 Jul 13;107(28):12692-7. doi: 10.1073/pnas.1002418107. Epub 2010 Jun 25. Proc Natl Acad Sci U S A. 2010. PMID: 20615999 Free PMC article.
-
Neurobiology of Pavlovian fear conditioning.Annu Rev Neurosci. 2001;24:897-931. doi: 10.1146/annurev.neuro.24.1.897. Annu Rev Neurosci. 2001. PMID: 11520922 Review.
-
An organization of visual and auditory fear conditioning in the lateral amygdala.Neurobiol Learn Mem. 2014 Dec;116:1-13. doi: 10.1016/j.nlm.2014.07.008. Epub 2014 Jul 27. Neurobiol Learn Mem. 2014. PMID: 25076183
-
Placing prediction into the fear circuit.Trends Neurosci. 2011 Jun;34(6):283-92. doi: 10.1016/j.tins.2011.03.005. Epub 2011 May 5. Trends Neurosci. 2011. PMID: 21549434 Free PMC article. Review.
Cited by
-
Between placebo and nocebo: Response to control treatment is mediated by amygdala activity and connectivity.Eur J Pain. 2020 Mar;24(3):580-592. doi: 10.1002/ejp.1510. Epub 2019 Dec 12. Eur J Pain. 2020. PMID: 31770471 Free PMC article.
-
Neural Computations Underlying Causal Structure Learning.J Neurosci. 2018 Aug 8;38(32):7143-7157. doi: 10.1523/JNEUROSCI.3336-17.2018. Epub 2018 Jun 29. J Neurosci. 2018. PMID: 29959234 Free PMC article.
-
The Basolateral Amygdalae and Frontotemporal Network Functions for Threat Perception.eNeuro. 2017 Mar 27;4(1):ENEURO.0314-16.2016. doi: 10.1523/ENEURO.0314-16.2016. eCollection 2017 Jan-Feb. eNeuro. 2017. PMID: 28374005 Free PMC article.
-
The role of prospective contingency in the control of behavior and dopamine signals during associative learning.bioRxiv [Preprint]. 2024 Feb 6:2024.02.05.578961. doi: 10.1101/2024.02.05.578961. bioRxiv. 2024. Update in: Nat Neurosci. 2025 Jun;28(6):1280-1292. doi: 10.1038/s41593-025-01915-4. PMID: 38370735 Free PMC article. Updated. Preprint.
-
Prospective contingency explains behavior and dopamine signals during associative learning.Nat Neurosci. 2025 Jun;28(6):1280-1292. doi: 10.1038/s41593-025-01915-4. Epub 2025 Mar 18. Nat Neurosci. 2025. PMID: 40102680 Free PMC article.
References
-
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat. Rev. Neurosci. 2004;5:844–852. - PubMed
-
- LeDoux JE. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–184. - PubMed
-
- Herry C, Johansen JP. Encoding of fear learning and memory in distributed neuronal circuits. Nat. Neurosci. 2014;17:1644–1654. - PubMed
-
- Gründemann J, Lüthi A. Ensemble coding in amygdala circuits for associative learning. Curr. Opin. Neurobiol. 2015;35:200–6. - PubMed
-
- Rescorla Ra. Probability of shock in the presence and absence of CS in fear conditioning. J. Comp. Physiol. Psychol. 1968;66:1–5. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

