Population-Based Colonoscopy Screening for Colorectal Cancer: A Randomized Clinical Trial
- PMID: 27214731
- PMCID: PMC5333856
- DOI: 10.1001/jamainternmed.2016.0960
Population-Based Colonoscopy Screening for Colorectal Cancer: A Randomized Clinical Trial
Abstract
Importance: Although some countries have implemented widespread colonoscopy screening, most European countries have not introduced it because of uncertainty regarding participation rates, procedure-related pain and discomfort, endoscopist performance, and effectiveness. To our knowledge, no randomized trials on colonoscopy screening currently exist.
Objective: To investigate participation rate, adenoma yield, performance, and adverse events of population-based colonoscopy screening in several European countries.
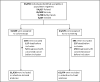
Design, setting, and population: A population-based randomized clinical trial was conducted among 94 959 men and women aged 55 to 64 years of average risk for colon cancer in Poland, Norway, the Netherlands, and Sweden from June 8, 2009, to June 23, 2014.
Interventions: Colonoscopy screening or no screening.
Main outcomes and measures: Participation in colonoscopy screening, cancer and adenoma yield, and participant experience. Study outcomes were compared by country and endoscopist.
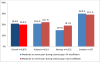
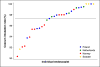
Results: Of 31 420 eligible participants randomized to the colonoscopy group, 12 574 (40.0%) underwent screening. Participation rates were 60.7% in Norway (5354 of 8816), 39.8% in Sweden (486 of 1222), 33.0% in Poland (6004 of 18 188), and 22.9% in the Netherlands (730 of 3194) (P < .001). The cecum intubation rate was 97.2% (12 217 of 12 574), with 9726 participants (77.4%) not receiving sedation. Of the 12 574 participants undergoing colonoscopy screening, we observed 1 perforation (0.01%), 2 postpolypectomy serosal burns (0.02%), and 18 cases of bleeding owing to polypectomy (0.14%). Sixty-two individuals (0.5%) were diagnosed with colorectal cancer and 3861 (30.7%) had adenomas, of which 1304 (10.4%) were high-risk adenomas. Detection rates were similar in the proximal and distal colon. Performance differed significantly between endoscopists; recommended benchmarks for cecal intubation (95%) and adenoma detection (25%) were not met by 6 (17.1%) and 10 of 35 endoscopists (28.6%), respectively. Moderate or severe abdominal pain after colonoscopy was reported by 601 of 3611 participants (16.7%) examined with standard air insufflation vs 214 of 5144 participants (4.2%) examined with carbon dioxide (CO2) insufflation (P < .001).
Conclusions and relevance: Colonoscopy screening entails high detection rates in the proximal and distal colon. Participation rates and endoscopist performance vary significantly. Postprocedure abdominal pain is common with standard air insufflation and can be significantly reduced by using CO2.
Trial registration: clinicaltrials.gov Identifier: NCT00883792.
Conflict of interest statement
Conflict of interest disclosure: Michael Bretthauer is member of the European scientific advisory board of Exact Sciences and has received equipment for testing in scientific studies from Olympus, Fujinon, Falk Pharma and CCS Healthcare. All other authors report no conflicts of interest.
Figures




Comment in
-
Colorectal Cancer Screening With Colonoscopy.JAMA Intern Med. 2016 Jul 1;176(7):903-4. doi: 10.1001/jamainternmed.2016.1333. JAMA Intern Med. 2016. PMID: 27214200 No abstract available.
References
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. - PubMed
-
- Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000;343:1603–7. - PubMed
-
- Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicenter randomized trial. Lancet. 2010;375:1624–33. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

