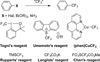
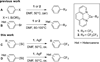
Trifluoromethylation of Arylsilanes with [(phen)CuCF3 ]
- PMID: 27214849
- PMCID: PMC4976628
- DOI: 10.1002/anie.201601163
Trifluoromethylation of Arylsilanes with [(phen)CuCF3 ]
Abstract
A method for the trifluoromethylation of arylsilanes is reported. The reaction proceeds with [(phen)CuCF3 ] as the CF3 source under mild, oxidative conditions with high functional-group compatibility. This transformation complements prior trifluoromethylation of arenes in several ways. Most important, this method converts arylsilanes formed by the silylation of aryl C-H bonds to trifluoromethylarenes, thereby allowing the conversion of arenes to trifluoromethylarenes. The unique capabilities of the reported method are demonstrated by the conversion of a C-H bond into a C-CF3 bond in active pharmaceutical ingredients which do not undergo this overall transformation by alternative functionalization processes, including a combination of borylation and trifluoromethylation.
Keywords: arenes; copper; cross-coupling; fluorine; silanes.
© 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
References
-
- Purser S, Moore PR, Swallow S, Gouverneur V. Chem. Soc. Rev. 2008;37:320–330. - PubMed
-
- Zhou Y, Wang J, Gu Z, Wang S, Zhu W, Aceña JL, Soloshonok VA, Izawa K, Liu H. Chem. Rev. 2016 - PubMed
-
- Swarts F. Bull. Acad. Roy. Belg. 1892;24:309.
-
- Petrov VA. Flourinated Heterocyclic Compounds: Synthesis, Chemistry, and Applications. Vol. 1. Weinheim: John Wiley & Sons; 2009.
-
- Chu L, Qing F-L. Org. Lett. 2010;12:5060–5063. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources