Tocilizumab in Autoimmune Encephalitis Refractory to Rituximab: An Institutional Cohort Study
- PMID: 27215218
- PMCID: PMC5081109
- DOI: 10.1007/s13311-016-0442-6
Tocilizumab in Autoimmune Encephalitis Refractory to Rituximab: An Institutional Cohort Study
Abstract
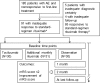
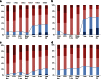
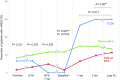
A considerable portion of autoimmune encephalitis (AE) does not respond to conventional immunotherapies and subsequently has poor outcomes. We aimed to determine the efficacy of tocilizumab, an anti-interleukin-6 antibody, in rituximab-refractory AE compared with other treatment options. From an institutional cohort of AE, 91 patients with inadequate clinical response to first-line immunotherapy and following rituximab were retrospectively reviewed. Patients were grouped according to their further immunotherapy strategies. Thirty (33.0 %) patients were included in the tocilizumab group, 31 (34.0 %) in the additional rituximab group, and 30 (33.0 %) in the observation group. Outcomes were defined as the favorable modified Rankin Scale scores (≤2) at 1 and 2 months from the initiation of each treatment strategy and at the last follow-up. Favorable clinical response (improvement of the modified Rankin Scale scores by ≥ 2 points or achievement of the mRS scores ≤ 2) at the last follow-up was also analyzed. The tocilizumab group showed more frequent favorable mRS scores at 2 months from treatment initiation and at the last follow-up compared with those at the relevant time points of the remaining groups. The majority (89.5 %) of the patients with clinical improvement at 1 month from tocilizumab treatment maintained a long-term favorable clinical response. No serious adverse effects of rituximab or tocilizumab were reported. Therefore, we suggest that tocilizumab might be a good treatment strategy for treating AE refractory to conventional immunotherapies and rituximab. The tocilizumab-mediated clinical improvement manifests as early at 1 month after treatment initiation.
Keywords: Autoimmune disorders; encephalitis; immunotherapy; prognosis; tocilizumab.
Conflict of interest statement
Compliance with Ethical Standards Conflicts of interest The authors have no conflicts of interest.
Figures
Comment in
-
Interleukin-6 Blockade as Rescue Therapy in Autoimmune Encephalitis.Neurotherapeutics. 2016 Oct;13(4):821-823. doi: 10.1007/s13311-016-0471-1. Neurotherapeutics. 2016. PMID: 27530677 Free PMC article. No abstract available.
References
-
- Gable MS, Sheriff H, Dalmau J, Tilley DH, Glaser CA. The frequency of autoimmune N-methyl-D-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the California Encephalitis Project. Clin Infect Dis. 2012;54:899–904. doi: 10.1093/cid/cir1038. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

