Total synthesis, biosynthesis and biological profiles of clavine alkaloids
- PMID: 27215547
- PMCID: PMC4917493
- DOI: 10.1039/c6ob00878j
Total synthesis, biosynthesis and biological profiles of clavine alkaloids
Abstract
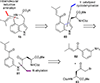
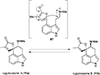
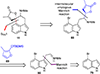
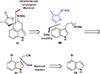
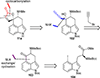
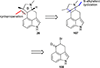
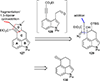
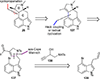
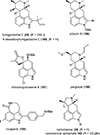
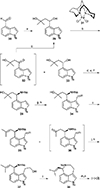
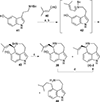
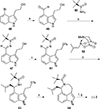
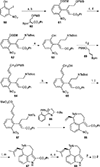
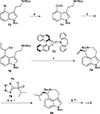
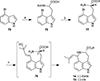
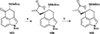
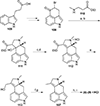
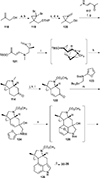
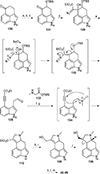
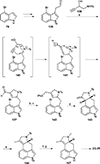
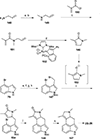
This review highlights noteworthy synthetic and biological aspects of the clavine subfamily of ergot alkaloids. Recent biosynthetic insights have laid the groundwork for a better understanding of the diverse biological pathways leading to these indole derivatives. Ergot alkaloids were among the first fungal-derived natural products identified, inspiring pharmaceutical applications in CNS disorders, migraine, infective diseases, and cancer. Pergolide, for example, is a semi-synthetic clavine alkaloid that has been used to treat Parkinson's disease. Synthetic activities have been particularly valuable to facilitate access to rare members of the Clavine family and empower medicinal chemistry research. Improved molecular target identification tools and a better understanding of signaling pathways can now be deployed to further extend the biological and medical utility of Clavine alkaloids.
Figures

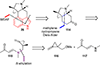

Similar articles
-
Ergot alkaloids: synthetic approaches to lysergic acid and clavine alkaloids.Nat Prod Rep. 2017 Apr 5;34(4):411-432. doi: 10.1039/c6np00110f. Nat Prod Rep. 2017. PMID: 28300233 Review.
-
Biosynthesis, total synthesis, and biological profiles of Ergot alkaloids.Alkaloids Chem Biol. 2021;85:1-112. doi: 10.1016/bs.alkal.2020.08.001. Epub 2020 Sep 30. Alkaloids Chem Biol. 2021. PMID: 33663751 Review.
-
Parasitic fungus Claviceps as a source for biotechnological production of ergot alkaloids.Biotechnol Adv. 2013 Jan-Feb;31(1):79-89. doi: 10.1016/j.biotechadv.2012.01.005. Epub 2012 Jan 12. Biotechnol Adv. 2013. PMID: 22261014 Review.
-
Ergot alkaloids: structure diversity, biosynthetic gene clusters and functional proof of biosynthetic genes.Nat Prod Rep. 2011 Mar;28(3):496-510. doi: 10.1039/c0np00060d. Epub 2010 Dec 24. Nat Prod Rep. 2011. PMID: 21186384 Review.
-
Post-genome research on the biosynthesis of ergot alkaloids.Planta Med. 2006 Oct;72(12):1117-20. doi: 10.1055/s-2006-947195. Epub 2006 Aug 10. Planta Med. 2006. PMID: 16902860 Review.
Cited by
-
Photoredox catalysis enabling decarboxylative radical cyclization of γ,γ-dimethylallyltryptophan (DMAT) derivatives: formal synthesis of 6,7-secoagroclavine.Beilstein J Org Chem. 2023 Jun 26;19:918-927. doi: 10.3762/bjoc.19.70. eCollection 2023. Beilstein J Org Chem. 2023. PMID: 37404801 Free PMC article.
-
Enzyme-Catalyzed Azepinoindole Formation in Clavine Alkaloid Biosynthesis.Org Lett. 2020 Apr 17;22(8):3302-3306. doi: 10.1021/acs.orglett.0c01132. Epub 2020 Apr 3. Org Lett. 2020. PMID: 32243182 Free PMC article.
-
Eight-Step Enantioselective Total Synthesis of (-)-Cycloclavine.Angew Chem Int Ed Engl. 2017 Jan 2;56(1):324-327. doi: 10.1002/anie.201608820. Epub 2016 Nov 18. Angew Chem Int Ed Engl. 2017. PMID: 27860203 Free PMC article.
-
Biological studies of clavine alkaloids targeting CNS receptors.Front Psychiatry. 2023 Nov 21;14:1286941. doi: 10.3389/fpsyt.2023.1286941. eCollection 2023. Front Psychiatry. 2023. PMID: 38076698 Free PMC article.
-
Concise Synthesis of (-)-Cycloclavine and (-)-5- epi-Cycloclavine via Asymmetric C-C Activation.J Am Chem Soc. 2018 Aug 1;140(30):9652-9658. doi: 10.1021/jacs.8b05549. Epub 2018 Jul 17. J Am Chem Soc. 2018. PMID: 29976068 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

