The Pharmacological Treatment of Chronic Obstructive Pulmonary Disease
- PMID: 27215595
- PMCID: PMC4961886
- DOI: 10.3238/arztebl.2016.0311
The Pharmacological Treatment of Chronic Obstructive Pulmonary Disease
Abstract
Background: Inhaled corticosteroids (ICS) are markedly less effective against chronic obstructive pulmonary disease (COPD) than against asthma, and also have worse side effects. Whether ICS should be used to treat COPD is currently a matter of debate.
Methods: This review is based on pertinent articles retrieved by a selective search in PubMed and the Excerpta Medica Database (EMBASE) carried out in May 2015. We analyzed clinical trials of ICS for the treatment of COPD with a duration of at least one year, along with meta-analyses and COPD guidelines.
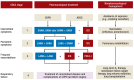
Results: ICS lower the frequency and severity of COPD exacerbations in comparison to monotherapy with a long-acting ß2-agonist, but have no effect on mortality. Compared to placebo, ICS monotherapy lessens the decline of forced expiratory volume in one second (FEV1) over one year by merely 5.80 mL (statistically insignificant; 95% confidence interval: [-0.28; 11.88]) and only marginally improve quality of life. ICS use in patients with COPD increases the risk of pneumonia. A combination of ICS with a long-acting bronchodilator improves FEV1 by 133 mL [105; 161] and lowers the frequency of severe exacerbations by 39% . The frequency of exacerbations is lowered mainly in patients who have many exacerbations; thus, ICS treatment is suitable only for patients with grade III or IV COPD.
Conclusion: ICS monotherapy has no clinically useful effect on pulmonary function in COPD. The main form of drug treatment for COPD is with broncho - dilators, either alone or in combination with ICS. ICS can be given to patients with grade III or IV COPD to make exacerbations less frequent. Patients with an asthma-COPD overlap syndrome (ACOS) can benefit from ICS treatment.
Figures
Comment in
-
Missing Information.Dtsch Arztebl Int. 2016 Oct 14;113(41):692. doi: 10.3238/arztebl.2016.0692a. Dtsch Arztebl Int. 2016. PMID: 27839541 Free PMC article. No abstract available.
Similar articles
-
Combination therapy of inhaled steroids and long-acting beta2-agonists in asthma-COPD overlap syndrome.Int J Chron Obstruct Pulmon Dis. 2016 Nov 8;11:2797-2803. doi: 10.2147/COPD.S114964. eCollection 2016. Int J Chron Obstruct Pulmon Dis. 2016. PMID: 27877033 Free PMC article.
-
Treatment trends in patients with asthma-COPD overlap syndrome in a COPD cohort: findings from a real-world survey.Int J Chron Obstruct Pulmon Dis. 2017 Jun 15;12:1753-1763. doi: 10.2147/COPD.S136314. eCollection 2017. Int J Chron Obstruct Pulmon Dis. 2017. PMID: 28670116 Free PMC article.
-
Effects of roflumilast in COPD patients receiving inhaled corticosteroid/long-acting β2-agonist fixed-dose combination: RE(2)SPOND rationale and study design.Int J Chron Obstruct Pulmon Dis. 2016 Aug 17;11:1921-8. doi: 10.2147/COPD.S109661. eCollection 2016. Int J Chron Obstruct Pulmon Dis. 2016. PMID: 27574416 Free PMC article. Clinical Trial.
-
Current evidence for COPD management with dual long-acting muscarinic antagonist/long-acting β2-agonist bronchodilators.Postgrad Med. 2020 Mar;132(2):198-205. doi: 10.1080/00325481.2019.1702834. Epub 2020 Jan 3. Postgrad Med. 2020. PMID: 31900019 Review.
-
Comparative safety and effectiveness of inhaled bronchodilators and corticosteroids for treating asthma-COPD overlap: a systematic review and meta-analysis.J Asthma. 2021 Mar;58(3):344-359. doi: 10.1080/02770903.2019.1687716. Epub 2019 Nov 12. J Asthma. 2021. PMID: 31668101
Cited by
-
Effect of Bufei Yishen Granules Combined with Electroacupuncture in Rats with Chronic Obstructive Pulmonary Disease via the Regulation of TLR-4/NF-κB Signaling.Evid Based Complement Alternat Med. 2019 May 29;2019:6708645. doi: 10.1155/2019/6708645. eCollection 2019. Evid Based Complement Alternat Med. 2019. PMID: 31275415 Free PMC article.
-
A Belgian survey on the diagnosis of asthma-COPD overlap syndrome.Int J Chron Obstruct Pulmon Dis. 2017 Feb 13;12:601-613. doi: 10.2147/COPD.S124459. eCollection 2017. Int J Chron Obstruct Pulmon Dis. 2017. PMID: 28243078 Free PMC article.
-
[Pulmonary involvement in cancers].Pneumologe (Berl). 2020;17(6):443-452. doi: 10.1007/s10405-020-00343-4. Epub 2020 Oct 13. Pneumologe (Berl). 2020. PMID: 33071699 Free PMC article. German.
-
In Reply.Dtsch Arztebl Int. 2016 Oct 14;113(41):692. doi: 10.3238/arztebl.2016.0692b. Dtsch Arztebl Int. 2016. PMID: 27839542 Free PMC article. No abstract available.
-
Management of Chronic Obstructive Pulmonary Disease.Arch Razi Inst. 2022 Feb 28;77(1):439-447. doi: 10.22092/ARI.2021.356613.1882. eCollection 2022 Feb. Arch Razi Inst. 2022. PMID: 35891752 Free PMC article.
References
-
- Committee GE. Global initiative for chronic obstructive lung disease. www.goldcopd.com. 2015 last accessed on 20 December 2015.
-
- Vestbo J, Sørensen T, Lange P, Brix A, Torre P, Viskum K. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 1999;355:1819–1823. - PubMed
-
- Pauwels RA, Lofdahl CG, Laitinen LA, et al. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. N Engl J Med. 1999;340:1948–1953. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

