Identification of Maturation-Specific Proteins by Single-Cell Proteomics of Human Oocytes
- PMID: 27215607
- PMCID: PMC4974340
- DOI: 10.1074/mcp.M115.056887
Identification of Maturation-Specific Proteins by Single-Cell Proteomics of Human Oocytes
Abstract
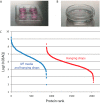
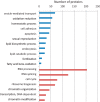
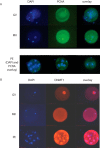
Oocytes undergo a range of complex processes via oogenesis, maturation, fertilization, and early embryonic development, eventually giving rise to a fully functioning organism. To understand proteome composition and diversity during maturation of human oocytes, here we have addressed crucial aspects of oocyte collection and proteome analysis, resulting in the first proteome and secretome maps of human oocytes. Starting from 100 oocytes collected via a novel serum-free hanging drop culture system, we identified 2,154 proteins, whose function indicate that oocytes are largely resting cells with a proteome that is tailored for homeostasis, cellular attachment, and interaction with its environment via secretory factors. In addition, we have identified 158 oocyte-enriched proteins (such as ECAT1, PIWIL3, NLRP7)(1) not observed in high-coverage proteomics studies of other human cell lines or tissues. Exploiting SP3, a novel technology for proteomic sample preparation using magnetic beads, we scaled down proteome analysis to single cells. Despite the low protein content of only ∼100 ng per cell, we consistently identified ∼450 proteins from individual oocytes. When comparing individual oocytes at the germinal vesicle (GV) and metaphase II (MII) stage, we found that the Tudor and KH domain-containing protein (TDRKH) is preferentially expressed in immature oocytes, while Wee2, PCNA, and DNMT1 were enriched in mature cells, collectively indicating that maintenance of genome integrity is crucial during oocyte maturation. This study demonstrates that an innovative proteomics workflow facilitates analysis of single human oocytes to investigate human oocyte biology and preimplantation development. The approach presented here paves the way for quantitative proteomics in other quantity-limited tissues and cell types. Data associated with this study are available via ProteomeXchange with identifier PXD004142.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- De La Fuente R., Viveiros M. M., Burns K. H., Adashi E. Y., Matzuk M. M., and Eppig J. J. (2004) Major chromatin remodeling in the germinal vesicle (GV) of mammalian oocytes is dispensable for global transcriptional silencing but required for centromeric heterochromatin function. Dev. Biol. 275, 447–458 - PubMed
-
- De La Fuente R., and Eppig J. J. (2001) Transcriptional activity of the mouse oocyte genome: Companion granulosa cells modulate transcription and chromatin remodeling. Dev. Biol. 229, 224–236 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

