Nitric oxide in paediatric respiratory disorders: novel interventions to address associated vascular phenomena?
- PMID: 27215618
- PMCID: PMC5942633
- DOI: 10.1177/1753944716649893
Nitric oxide in paediatric respiratory disorders: novel interventions to address associated vascular phenomena?
Abstract
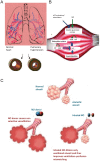
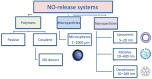
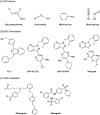
Nitric oxide (NO) has a significant role in modulating the respiratory system and is being exploited therapeutically. Neonatal respiratory failure can affect around 2% of all live births and is responsible for over one third of all neonatal mortality. Current treatment method with inhaled NO (iNO) has demonstrated great benefits to patients with persistent pulmonary hypertension, bronchopulmonary dysplasia and neonatal respiratory distress syndrome. However, it is not without its drawbacks, which include the need for patients to be attached to mechanical ventilators. Notably, there is also a lack of identification of subgroups amongst abovementioned patients, and homogeneity in powered studies associated with iNO, which is one of the limitations. There are significant developments in drug delivery methods and there is a need to look at alternative or supplementary methods of NO delivery that could reduce current concerns. The addition of NO-independent activators and stimulators, or drugs such as prostaglandins to work in synergy with NO donors might be beneficial. It is of interest to consider such delivery methods within the respiratory system, where controlled release of NO can be introduced whilst minimizing the production of harmful byproducts. This article reviews current therapeutic application of iNO and the state-of-the-art technology methods for sustained delivery of NO that may be adapted and developed to address respiratory disorders. We envisage this perspective would prompt active investigation of such systems for their potential clinical benefit.
Keywords: inhaled nitric oxide; nitric oxide; pulmonary; respiratory; vascular.
© The Author(s), 2016.
Conflict of interest statement
Figures


Similar articles
-
Nitric oxide for the treatment of preterm infants with respiratory distress syndrome.Expert Opin Pharmacother. 2013 Jan;14(1):97-103. doi: 10.1517/14656566.2013.746662. Epub 2012 Nov 30. Expert Opin Pharmacother. 2013. PMID: 23194109 Review.
-
Inhaled nitric oxide therapy in premature newborns.Curr Opin Pediatr. 2006 Apr;18(2):107-11. doi: 10.1097/01.mop.0000193291.09894.6c. Curr Opin Pediatr. 2006. PMID: 16601487 Review.
-
Inhaled nitric oxide for respiratory failure in preterm infants.Neonatology. 2012;102(4):251-3. doi: 10.1159/000338552. Epub 2012 Aug 15. Neonatology. 2012. PMID: 22907671 Review.
-
[Persistent pulmonary hypertension in premature and newborn infants: selective pulmonary vasodilation with inhaled nitric oxide (iNO)].Z Geburtshilfe Neonatol. 1998 Jan-Feb;202(1):25-9. Z Geburtshilfe Neonatol. 1998. PMID: 9577919 German.
-
Inhaled nitric oxide therapy of pulmonary hypertension and respiratory failure in premature and term neonates.Adv Pharmacol. 1995;34:457-74. doi: 10.1016/s1054-3589(08)61103-5. Adv Pharmacol. 1995. PMID: 8562452 Review. No abstract available.
Cited by
-
Molsidomine decreases hyperoxia-induced lung injury in neonatal rats.Pediatr Res. 2023 Oct;94(4):1341-1348. doi: 10.1038/s41390-023-02643-w. Epub 2023 May 13. Pediatr Res. 2023. PMID: 37179436
-
Critical role of nitric oxide in impeding COVID-19 transmission and prevention: a promising possibility.Environ Sci Pollut Res Int. 2022 Jun;29(26):38657-38672. doi: 10.1007/s11356-022-19148-4. Epub 2022 Mar 8. Environ Sci Pollut Res Int. 2022. PMID: 35258738 Free PMC article. Review.
-
The Potential Role of Nitric Oxide as a Therapeutic Agent against SARS-CoV-2 Infection.Int J Mol Sci. 2023 Dec 5;24(24):17162. doi: 10.3390/ijms242417162. Int J Mol Sci. 2023. PMID: 38138990 Free PMC article. Review.
-
Dual-acting biofunctionalised scaffolds for applications in regenerative medicine.J Mater Sci Mater Med. 2017 Feb;28(2):32. doi: 10.1007/s10856-017-5849-z. Epub 2017 Jan 20. J Mater Sci Mater Med. 2017. PMID: 28108960
References
-
- Adhikari K., Dellinger R., Lundin S. (2014) Inhaled nitric oxide does not reduce mortality in patients with acute respiratory distress syndrome regardless of severity: systematic review and metanalysis. Crit Care Med 42: 404–412. - PubMed
-
- Afshari A., Brok J., Moller A., Wetterslev J. (2011) Inhaled nitric oxide for acute respiratory distress syndrome and acute lung injury in adults and children: a systematic review with meta-analysis and trial sequential analysis. Anesth Analg 112: 1411–1421. - PubMed
-
- Bai S., Ahsan F. (2009) Synthesis and evaluation of pegylated dendrimeric nanocarrier for pulmonary delivery of low molecular weight heparin. Pharm Res 26: 539–548. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

