A compact light-sheet microscope for the study of the mammalian central nervous system
- PMID: 27215692
- PMCID: PMC4877654
- DOI: 10.1038/srep26317
A compact light-sheet microscope for the study of the mammalian central nervous system
Abstract
Investigation of the transient processes integral to neuronal function demands rapid and high-resolution imaging techniques over a large field of view, which cannot be achieved with conventional scanning microscopes. Here we describe a compact light sheet fluorescence microscope, featuring a 45° inverted geometry and an integrated photolysis laser, that is optimized for applications in neuroscience, in particular fast imaging of sub-neuronal structures in mammalian brain slices. We demonstrate the utility of this design for three-dimensional morphological reconstruction, activation of a single synapse with localized photolysis, and fast imaging of neuronal Ca(2+) signalling across a large field of view. The developed system opens up a host of novel applications for the neuroscience community.
Conflict of interest statement
The intellectual property rights contained in this publication are covered by patent applications which are licensed to M Squared Lasers Limited, Glasgow, UK.
Figures

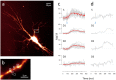
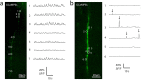

References
-
- Huisken J., Swoger J., Del Bene F., Wittbrodt J. & Stelzer E. H. K. Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science 305, 1007–1009 (2004). - PubMed
-
- Ahrens M. B., Orger M. B., Robson D. N., Li J. M. & Keller P. J. Whole-brain functional imaging at cellular resolution using light-sheet microscopy. Nat. Methods 10, 413–420 (2013). - PubMed
-
- Vladimirov N. et al. Light-sheet functional imaging in fictively behaving zebrafish. Nat. Methods 11, 883–884 (2014). - PubMed
-
- Dodt H.-U. et al. Ultramicroscopy: three-dimensional visualization of neuronal networks in the whole mouse brain. Nat. Methods 4, 331–336 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

