Optical electrophysiology for probing function and pharmacology of voltage-gated ion channels
- PMID: 27215841
- PMCID: PMC4907688
- DOI: 10.7554/eLife.15202
Optical electrophysiology for probing function and pharmacology of voltage-gated ion channels
Abstract
Voltage-gated ion channels mediate electrical dynamics in excitable tissues and are an important class of drug targets. Channels can gate in sub-millisecond timescales, show complex manifolds of conformational states, and often show state-dependent pharmacology. Mechanistic studies of ion channels typically involve sophisticated voltage-clamp protocols applied through manual or automated electrophysiology. Here, we develop all-optical electrophysiology techniques to study activity-dependent modulation of ion channels, in a format compatible with high-throughput screening. Using optical electrophysiology, we recapitulate many voltage-clamp protocols and apply to Nav1.7, a channel implicated in pain. Optical measurements reveal that a sustained depolarization strongly potentiates the inhibitory effect of PF-04856264, a Nav1.7-specific blocker. In a pilot screen, we stratify a library of 320 FDA-approved compounds by binding mechanism and kinetics, and find close concordance with patch clamp measurements. Optical electrophysiology provides a favorable tradeoff between throughput and information content for studies of NaV channels, and possibly other voltage-gated channels.
Keywords: biophysics; electrophysiology; high throughput screening; ion channels; neuroscience; none; optogenetics; structural biology.
Conflict of interest statement
AEC: A co-founder of Q-State Biosciences.
The other authors declare that no competing interests exist.
Figures


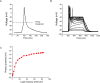

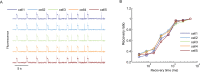







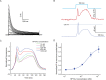
References
-
- Agus V, Di Silvio A, Rolland JF, Mondini A, Tremolada S, Montag K, Scarabottolo L, Redaelli L, Lohmer S. Bringing the light to high throughput screening: use of optogenetic tools for the development of recombinant cellular assays. Proc. SPIE. 2015;9303:e15202. doi: 10.1117/12.2077579. - DOI
-
- Ahuja S, Mukund S, Deng L, Khakh K, Chang E, Ho H, Shriver S, Young C, Lin S, Johnson JP, Wu P, Li J, Coons M, Tam C, Brillantes B, Sampang H, Mortara K, Bowman KK, Clark KR, Estevez A, Xie Z, Verschoof H, Grimwood M, Dehnhardt C, Andrez JC, Focken T, Sutherlin DP, Safina BS, Starovasnik MA, Ortwine DF, Franke Y, Cohen CJ, Hackos DH, Koth CM, Payandeh J. Structural basis of Nav1.7 inhibition by an isoform-selective small-molecule antagonist. Science. 2015;350:aac5464. doi: 10.1126/science.aac5464. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

