Design and Synthesis of Potent in Vitro and in Vivo Anticancer Agents Based on 1-(3',4',5'-Trimethoxyphenyl)-2-Aryl-1H-Imidazole
- PMID: 27216165
- PMCID: PMC4877593
- DOI: 10.1038/srep26602
Design and Synthesis of Potent in Vitro and in Vivo Anticancer Agents Based on 1-(3',4',5'-Trimethoxyphenyl)-2-Aryl-1H-Imidazole
Abstract
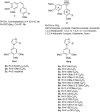
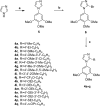
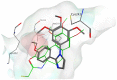
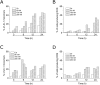
A novel series of tubulin polymerization inhibitors, based on the 1-(3',4',5'-trimethoxyphenyl)-2-aryl-1H-imidazole scaffold and designed as cis-restricted combretastatin A-4 analogues, was synthesized with the goal of evaluating the effects of various patterns of substitution on the phenyl at the 2-position of the imidazole ring on biological activity. A chloro and ethoxy group at the meta- and para-positions, respectively, produced the most active compound in the series (4o), with IC50 values of 0.4-3.8 nM against a panel of seven cancer cell lines. Except in HL-60 cells, 4o had greater antiproliferative than CA-4, indicating that the 3'-chloro-4'-ethoxyphenyl moiety was a good surrogate for the CA-4 B-ring. Experiments carried out in a mouse syngenic model demonstrated high antitumor activity of 4o, which significantly reduced the tumor mass at a dose thirty times lower than that required for CA-4P, which was used as a reference compound. Altogether, our findings suggest that 4o is a promising anticancer drug candidate that warrants further preclinical evaluation.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
-
- Nepali K., Ojha R., Sharma S., Bedi P. M. S. & Dhar K. L. Tubulin inhibitors: a patent survey. Recent Pat. Anticancer Drug Discov. 9, 1–45 (2014). - PubMed
-
- Pettit G. R. et al. Isolation and structure of the strong cell growth and tubulin inhibitor combretastatin A-4. Experientia 45, 209–211 (1989). - PubMed
-
- Lin C. M., Ho H. H., Pettit G. R. & Hamel E. Antimitotic natural products combretastatin A-4 and combretastatin A-2: studies on the mechanism of their inhibition of the binding of colchicine to tubulin. Biochemistry 28, 6984–6991 (1989). - PubMed
-
- McGown A. T. & Fox B. W. Differential cytotoxicity of combretastatins A1 and A4 in two daunorubucin-resistant P388 cell lines. Cancer Chemother. Pharmacol. 26, 79–81 (1990). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

